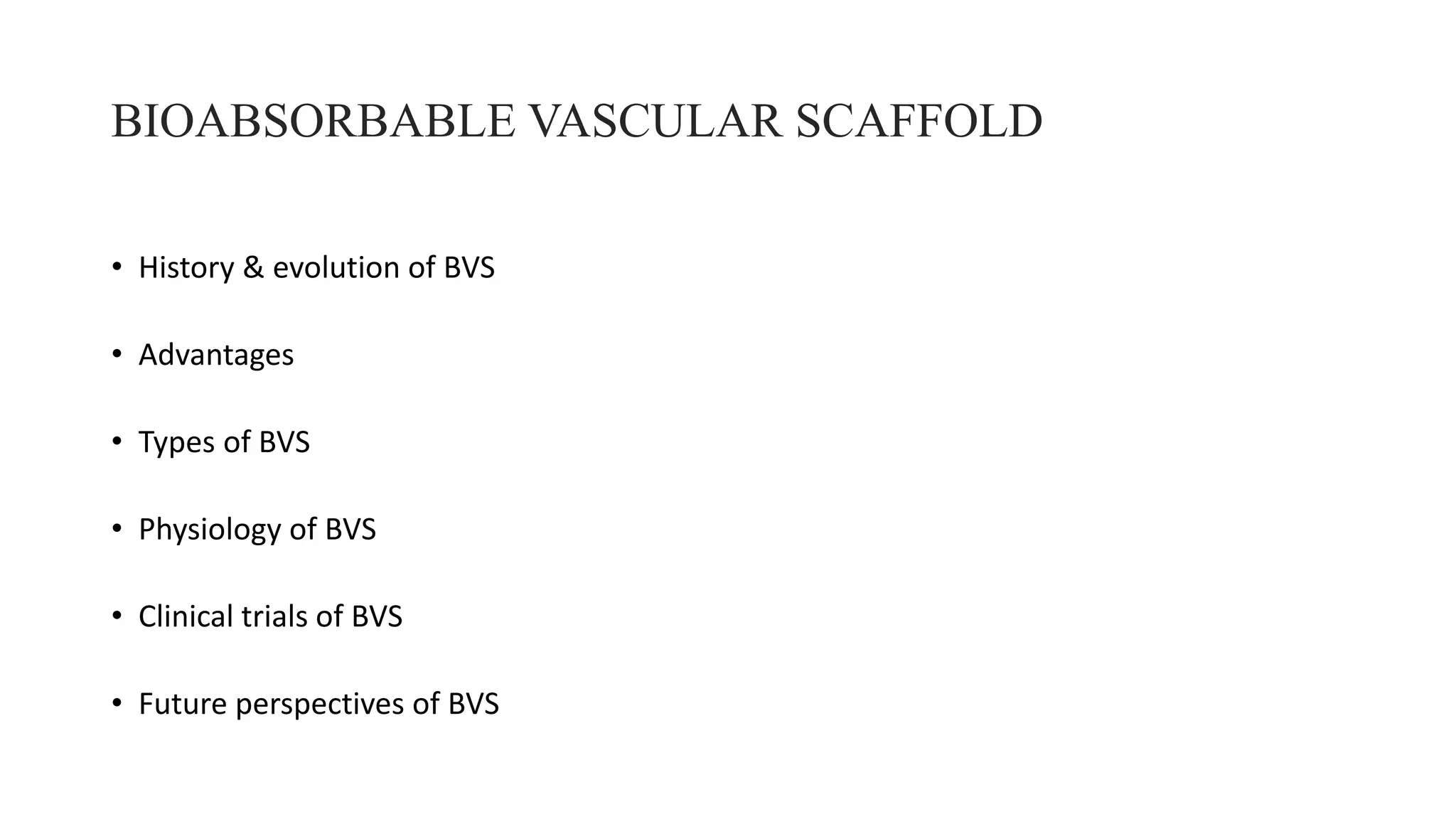
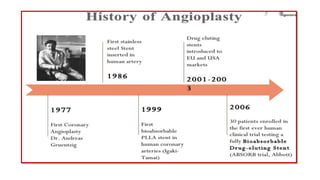
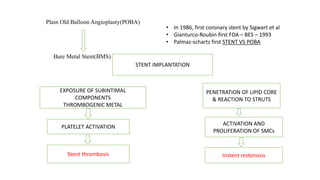
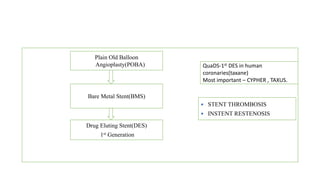
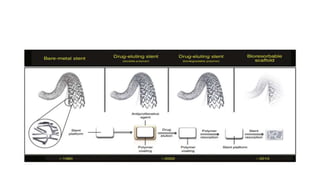
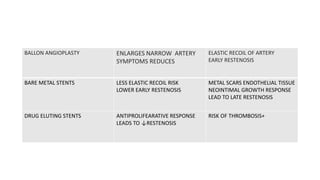
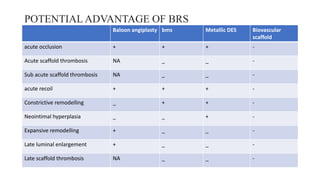
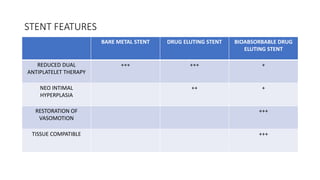
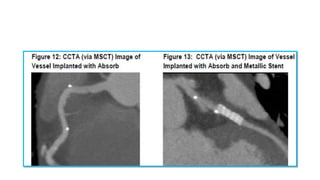
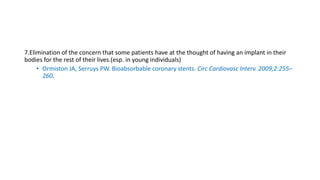
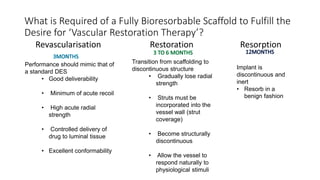
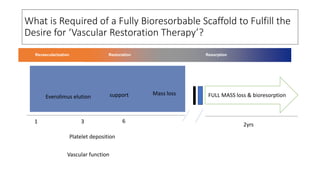
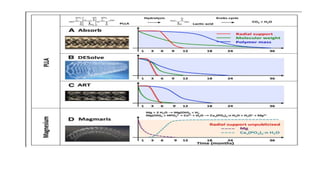
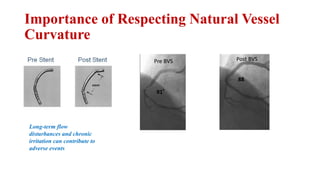
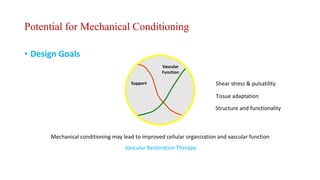
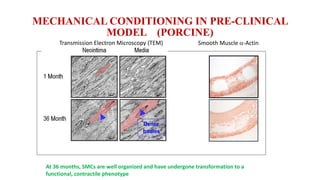
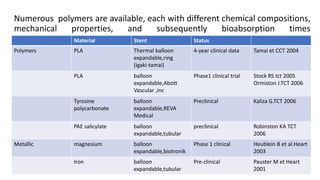
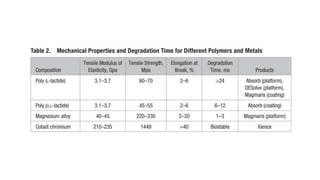
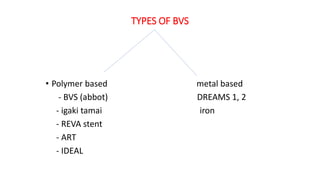
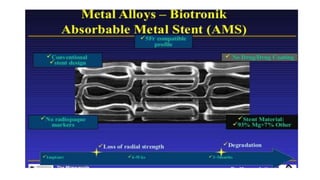
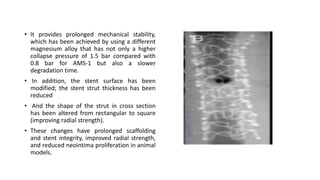
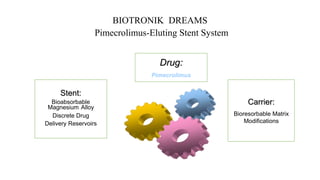
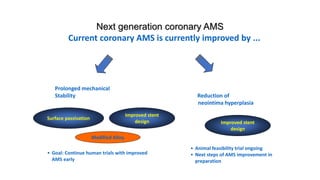
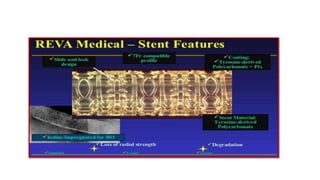
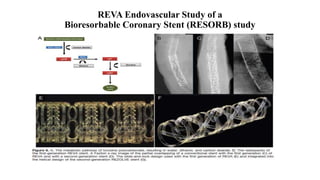
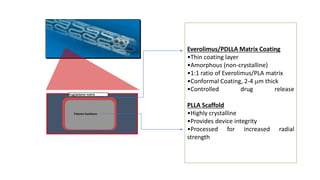
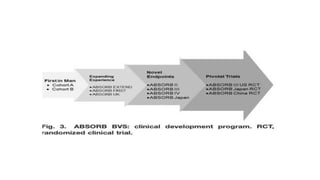
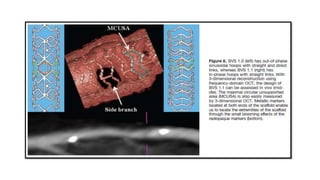
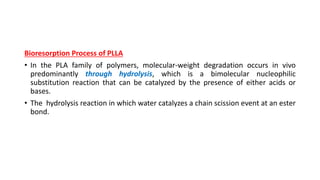
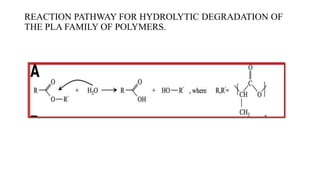
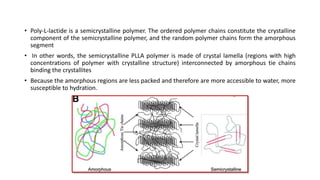
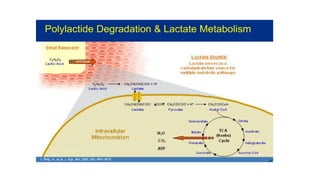
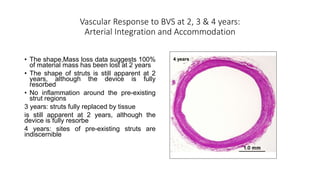
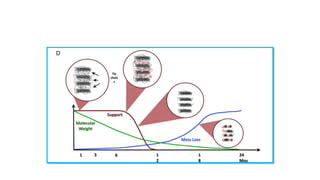
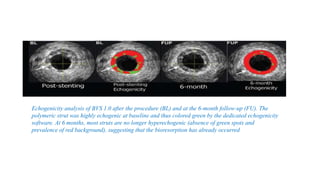
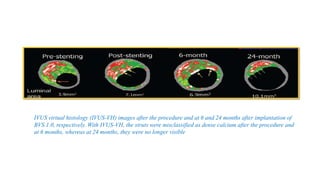
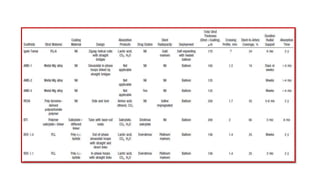
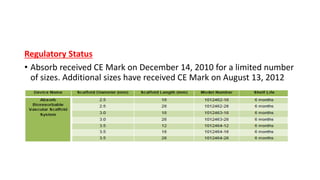
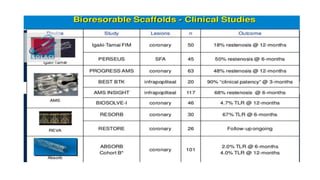
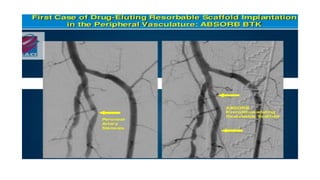
The document discusses the evolution of bioabsorbable vascular scaffolds (BVS) and their advantages compared to traditional angioplasty and stents such as bare metal stents (BMS) and drug-eluting stents (DES). It highlights the history of interventions, their benefits like reduced complications from long-term implants, and the potential for improved vascular remodeling and patient treatment outcomes. Additionally, it explores the development of BVS technology, its phases, and various types of BVS currently in use.