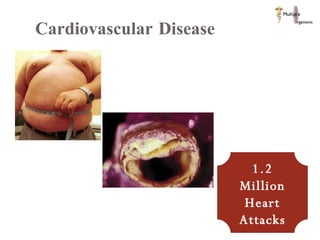
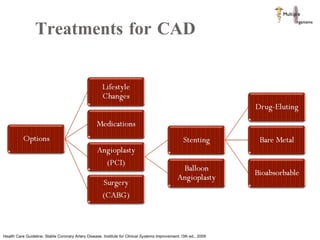
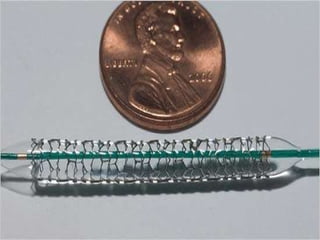
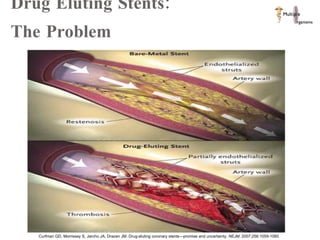
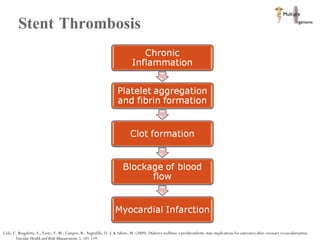
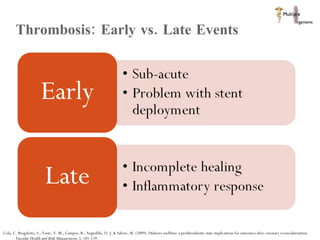
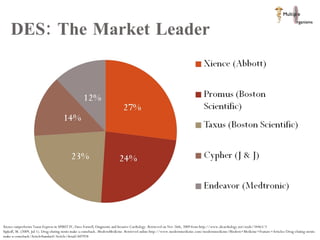
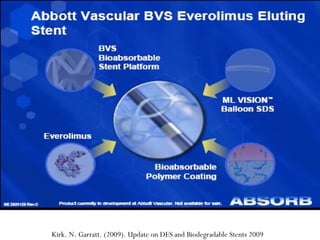
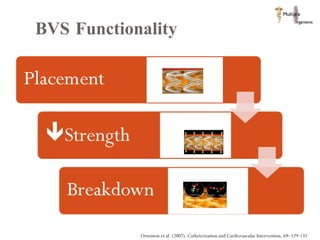
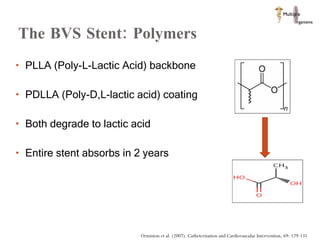
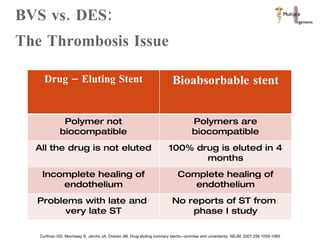
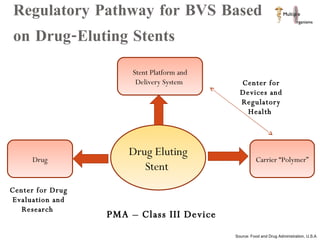
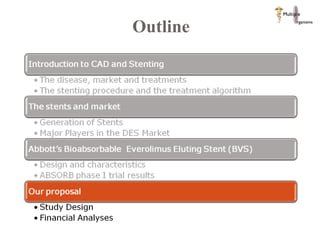
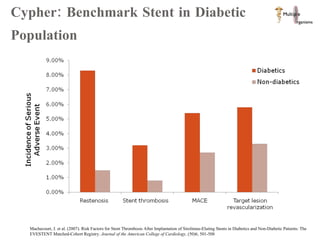
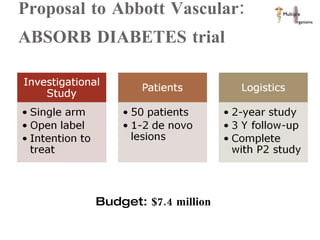
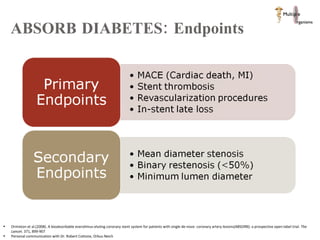
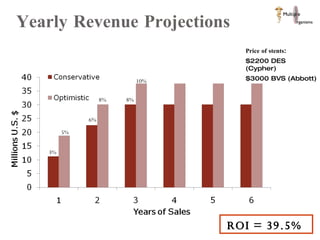
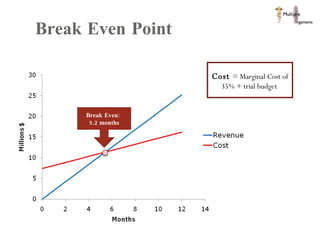
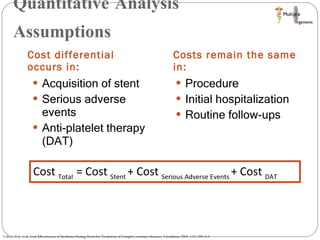
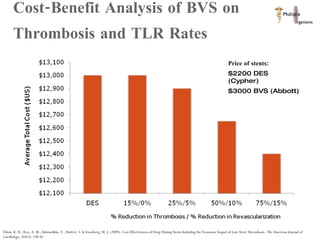
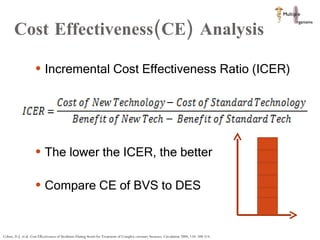
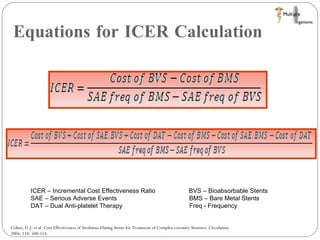
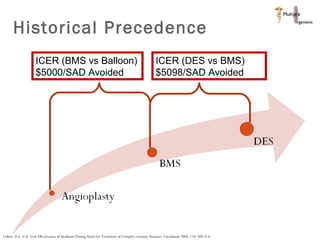
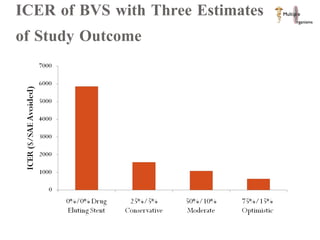
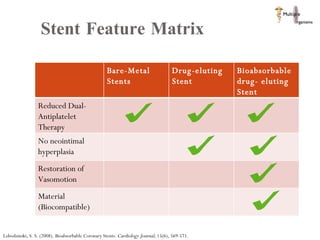
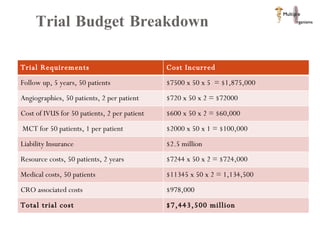
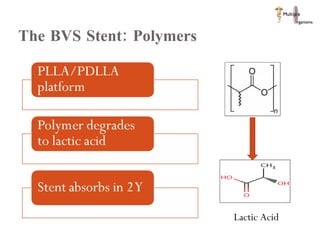
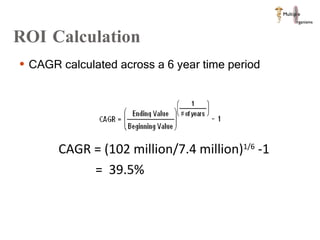
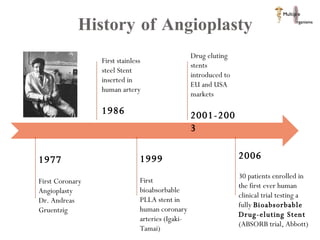
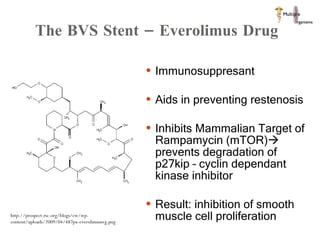
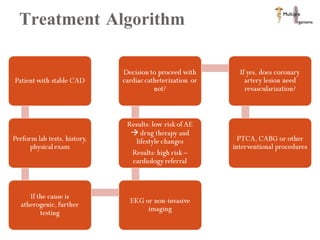
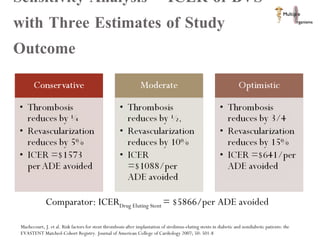
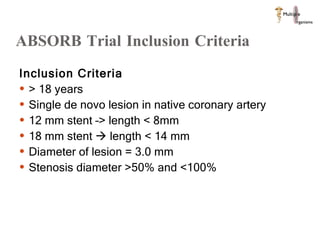
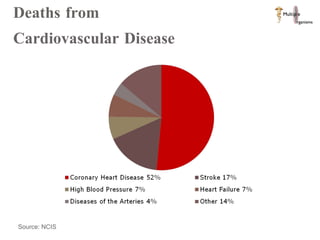
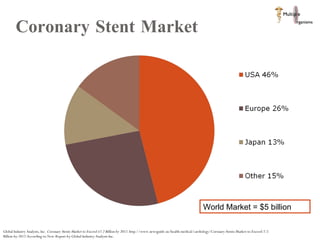
The document discusses Abbott's bioabsorbable everolimus-eluting stent and its potential advantages over drug-eluting stents. It proposes a clinical trial to test the bioabsorbable stent in diabetic patients to demonstrate improved safety and efficacy compared to drug-eluting stents in a high risk population. Quantitative analyses estimate the cost-effectiveness and return on investment of the bioabsorbable stent if thrombosis and revascularization rates are reduced compared to drug-eluting stents.