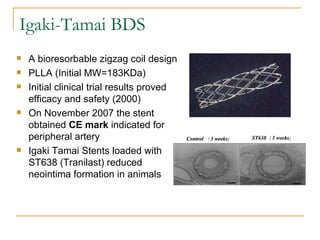
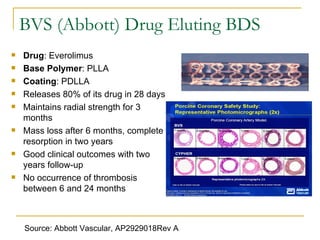
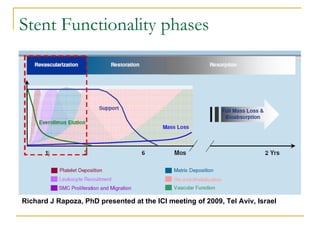
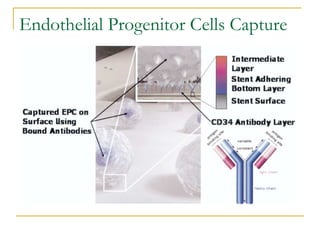
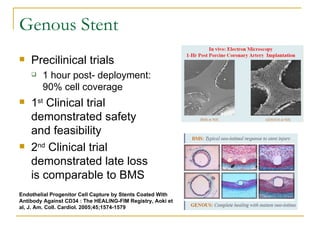
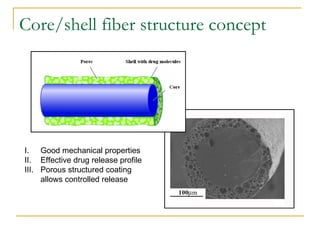
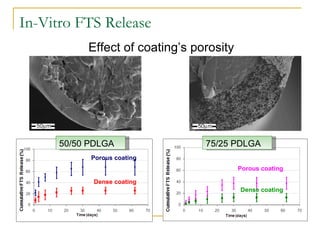
This document discusses biodegradable coronary stents and future solutions. It notes that metal stents have drawbacks like permanent irritation and inability to restore normal vessel physiology. Biodegradable stents aim to eliminate these issues by degrading over time and allowing the vessel to heal. Design considerations for biodegradable stents include degradation rate, biocompatibility, mechanical properties, and drug loading. Examples of biodegradable stents discussed include the Igaki-Tamai stent, Abbott's everolimus-eluting stent, and REVA's paclitaxel-eluting stent. New concepts discussed are pro-healing stents coated with endothelial progenitor cells and drug-eluting ballo