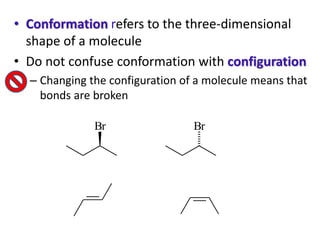
This document provides an overview of the topics that will be covered in the conformational analysis chemistry module, including:
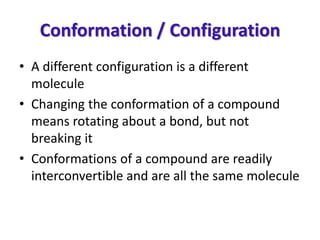
- The differences between conformation and configuration
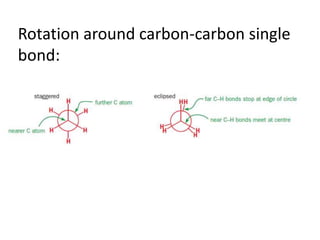
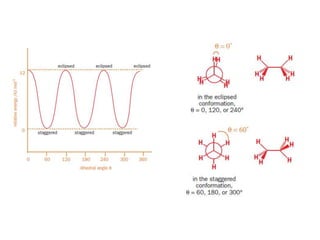
- Rotation around single carbon-carbon bonds
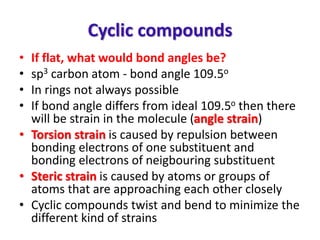
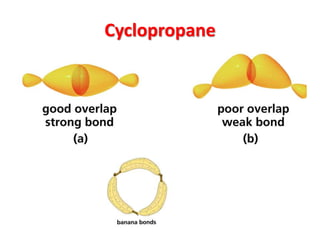
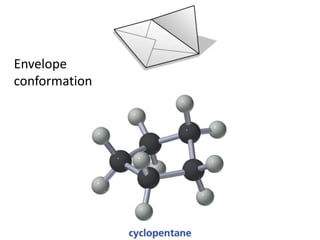
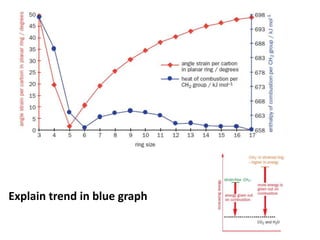
- Sources of strain in cyclic compounds and how their structures minimize this strain
- The chair and boat conformations of cyclohexane and factors that determine stability
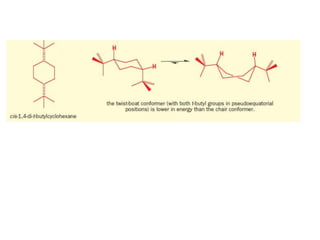
- The different conformations of monosubstituted and disubstituted cyclohexanes
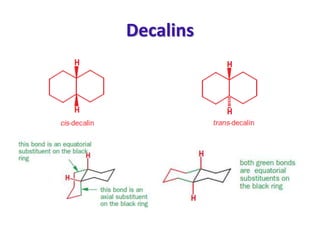
- Fused ring systems and decalins
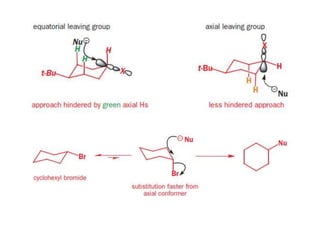
- Elimination reactions in cyclic compounds
Students are instructed to obtain molecular models to aid in visualizing the 3D structures discussed.