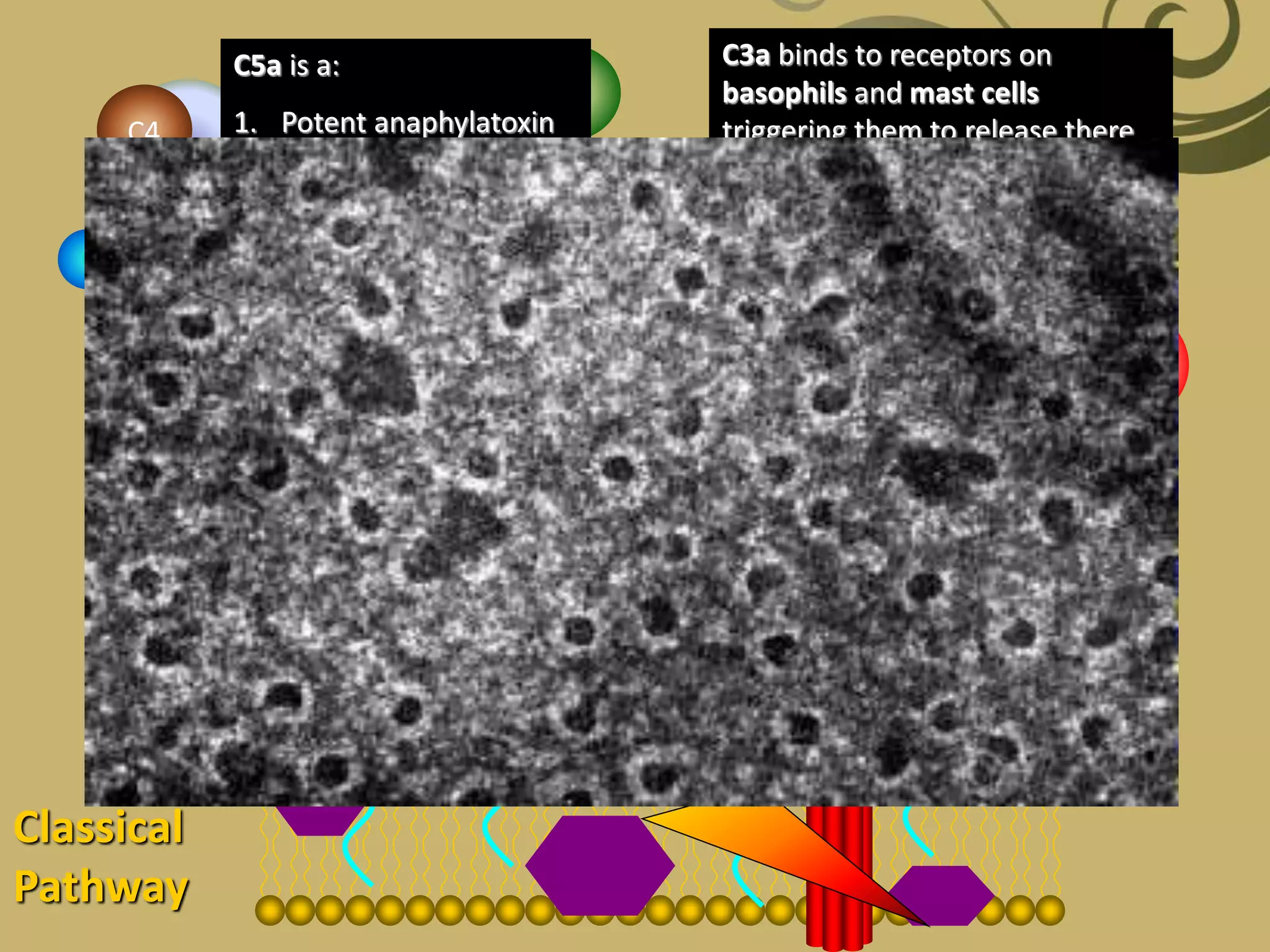
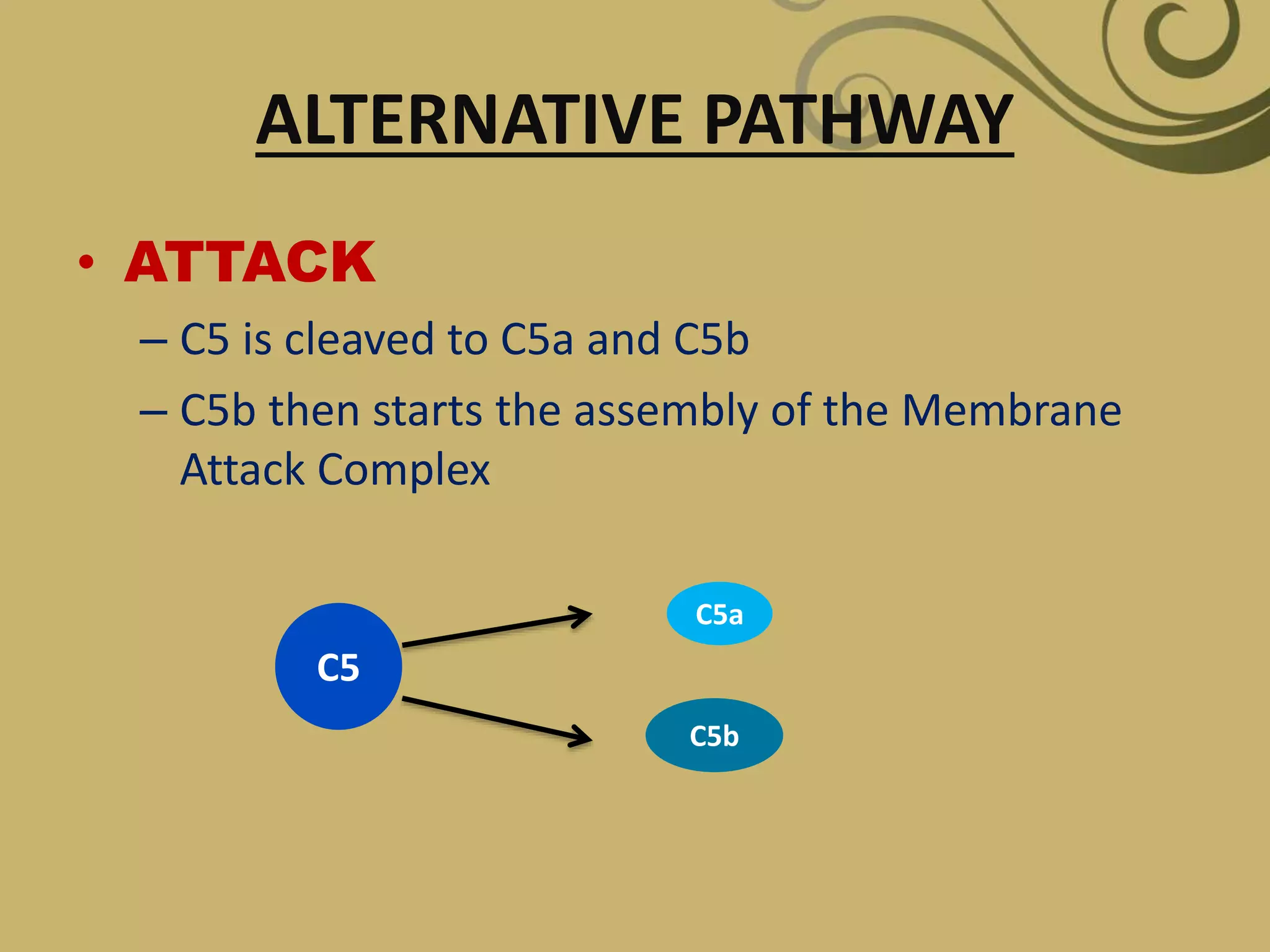
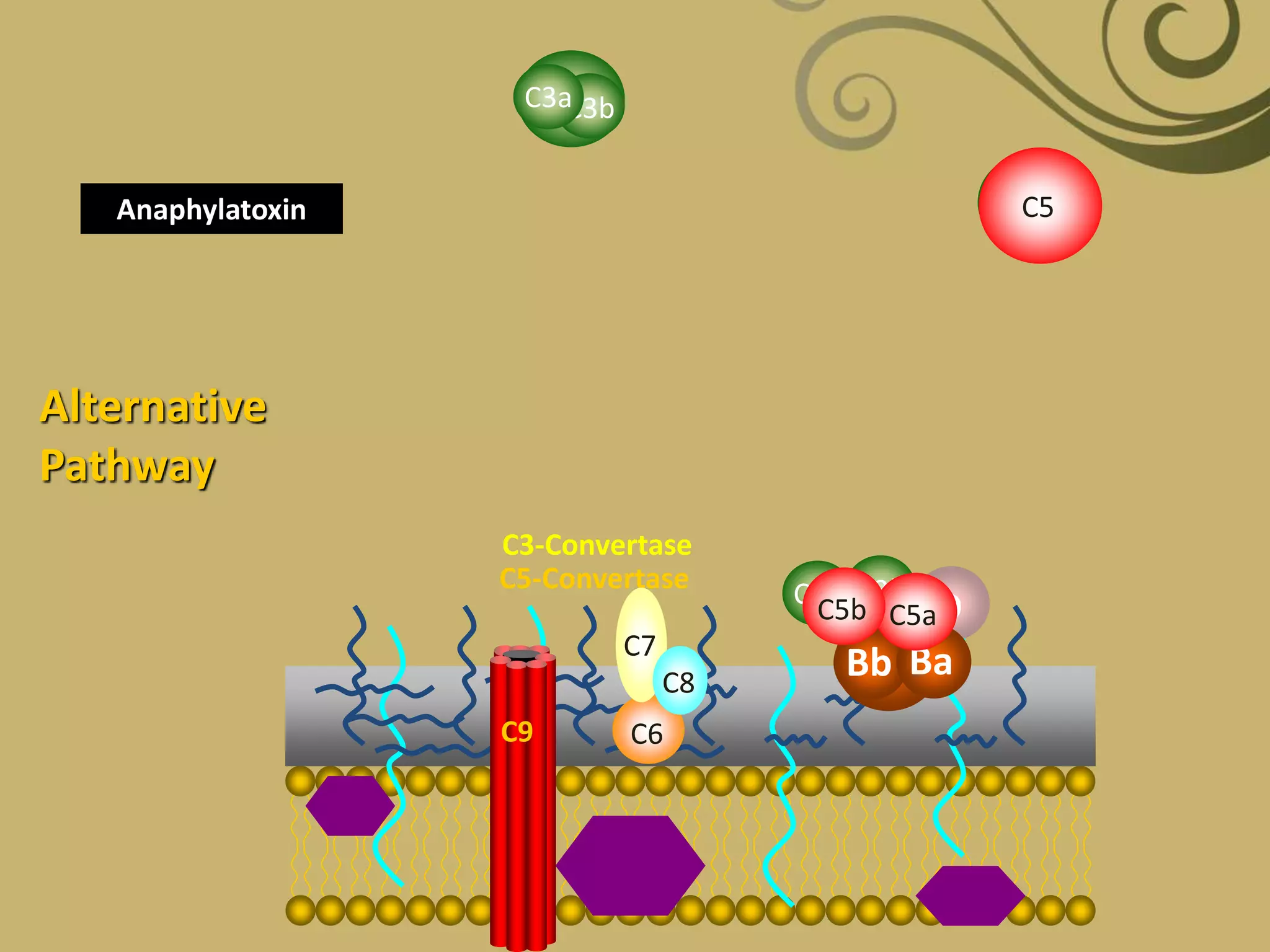
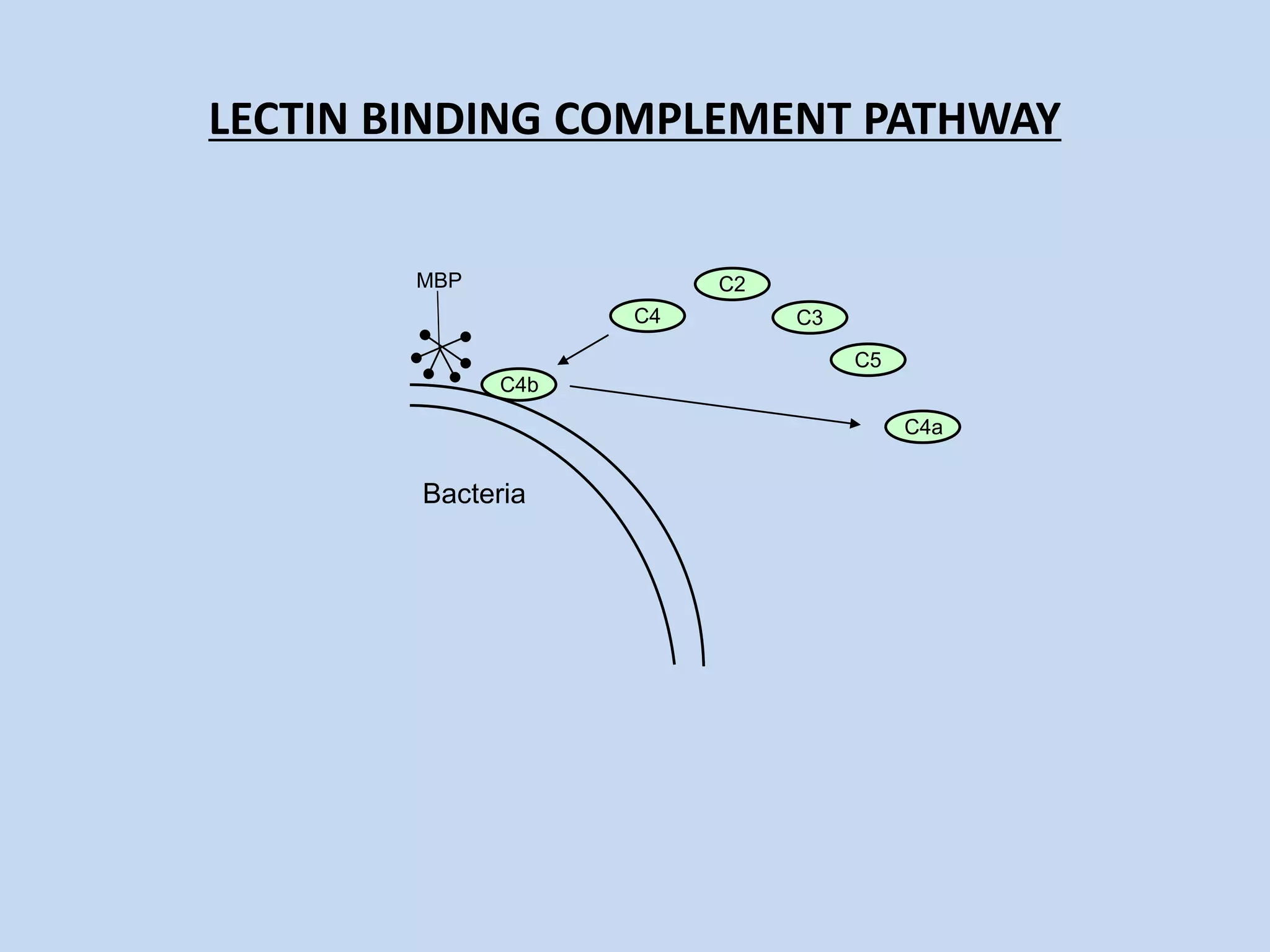
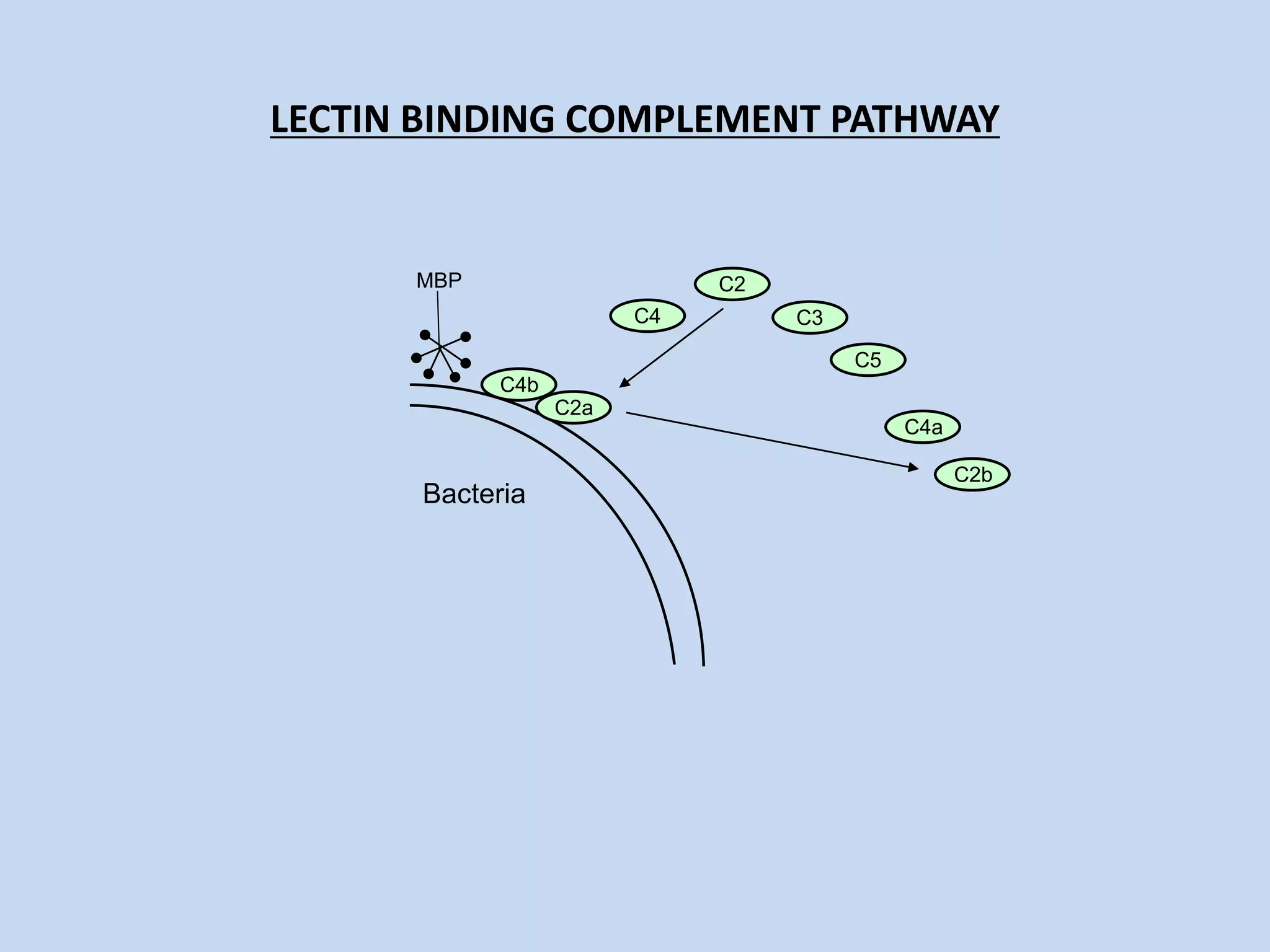
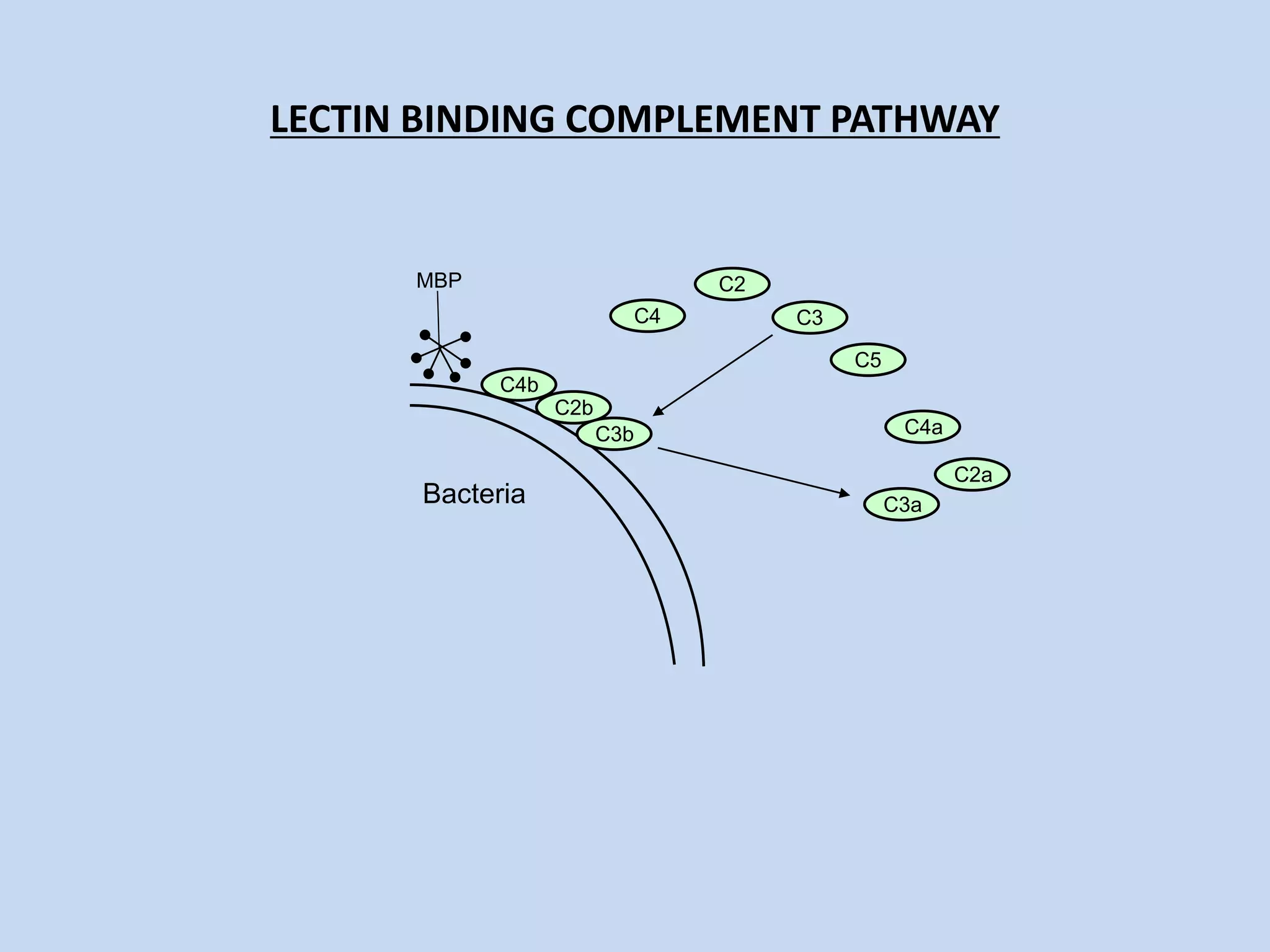
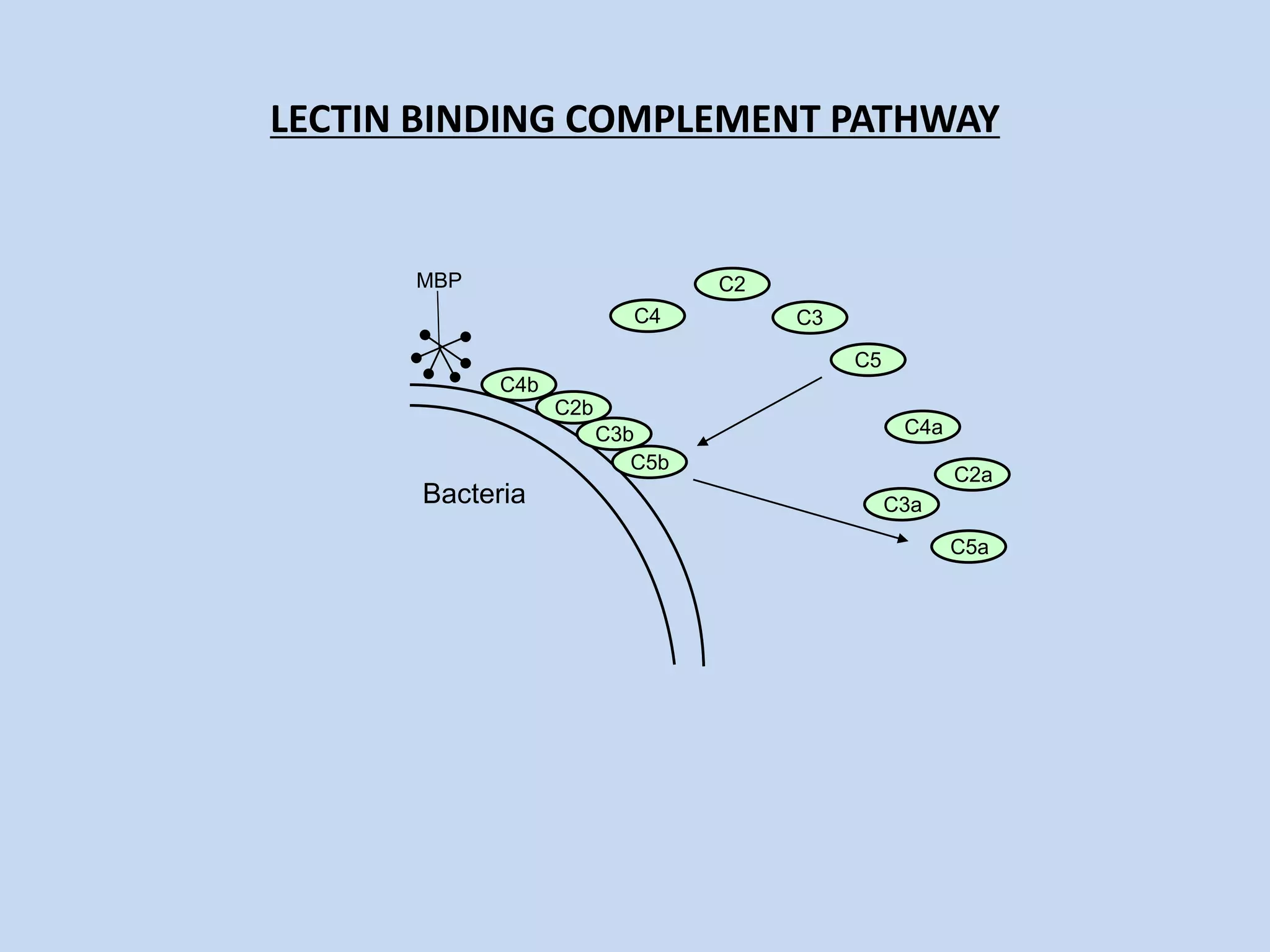
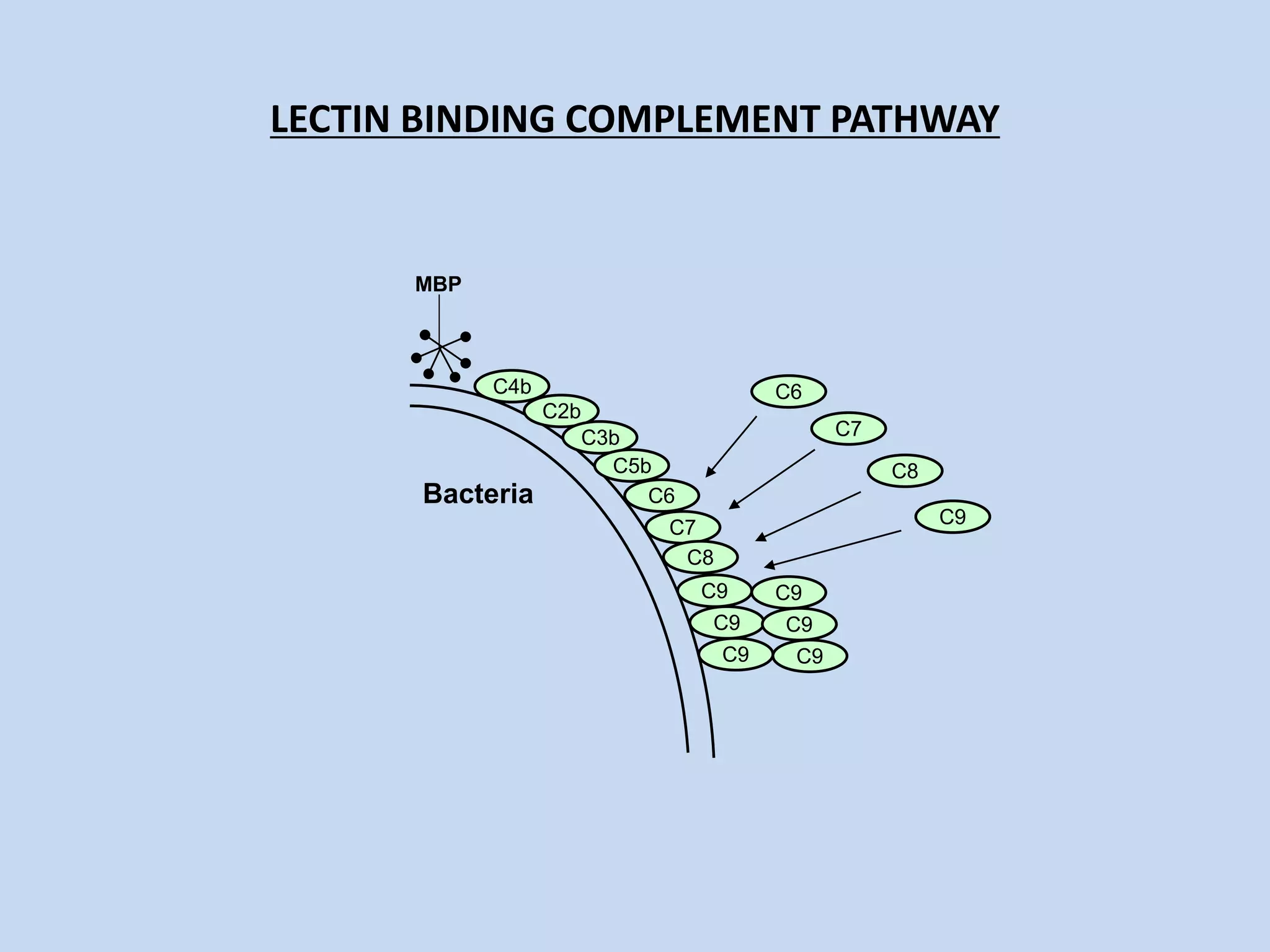
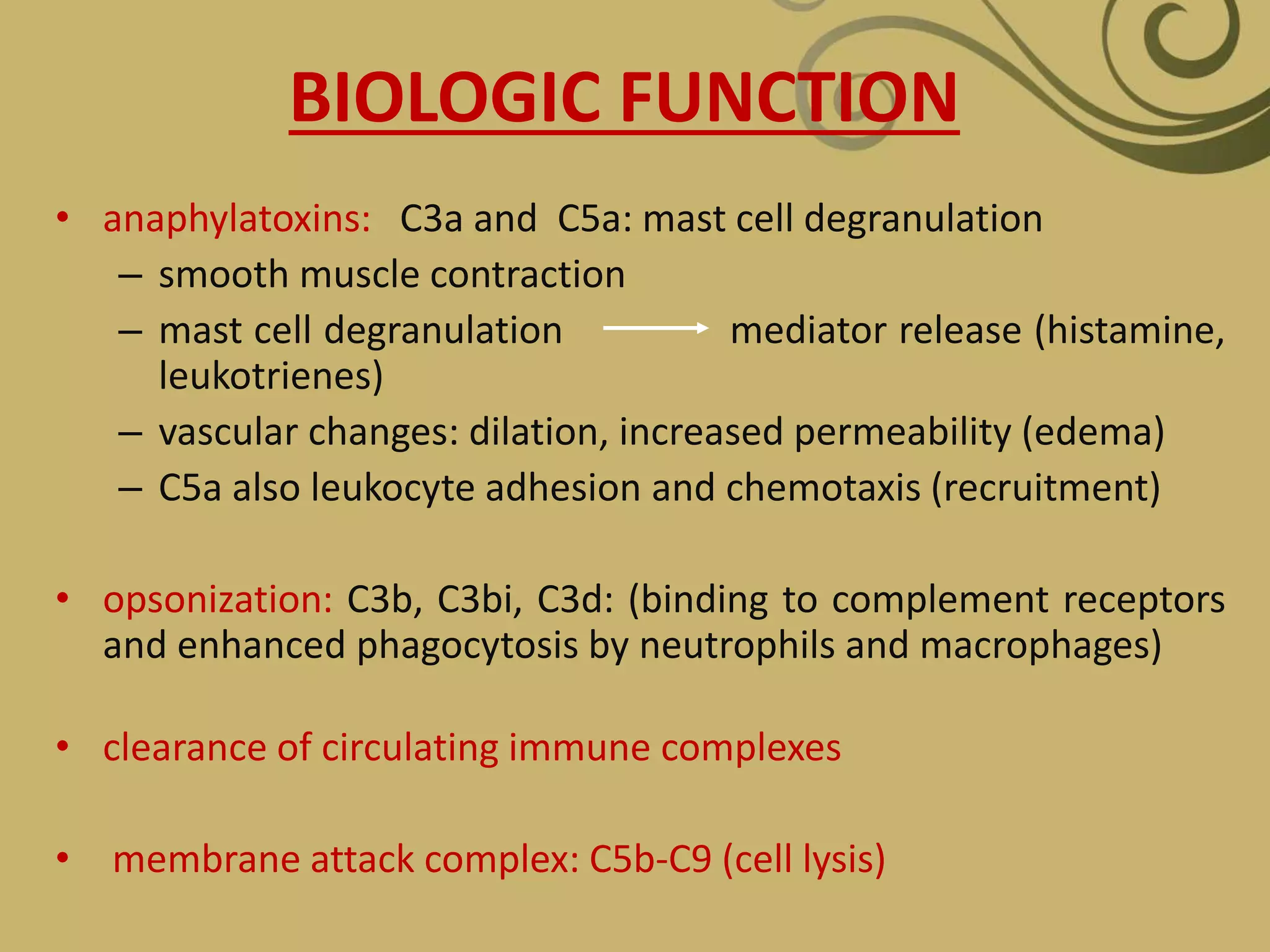
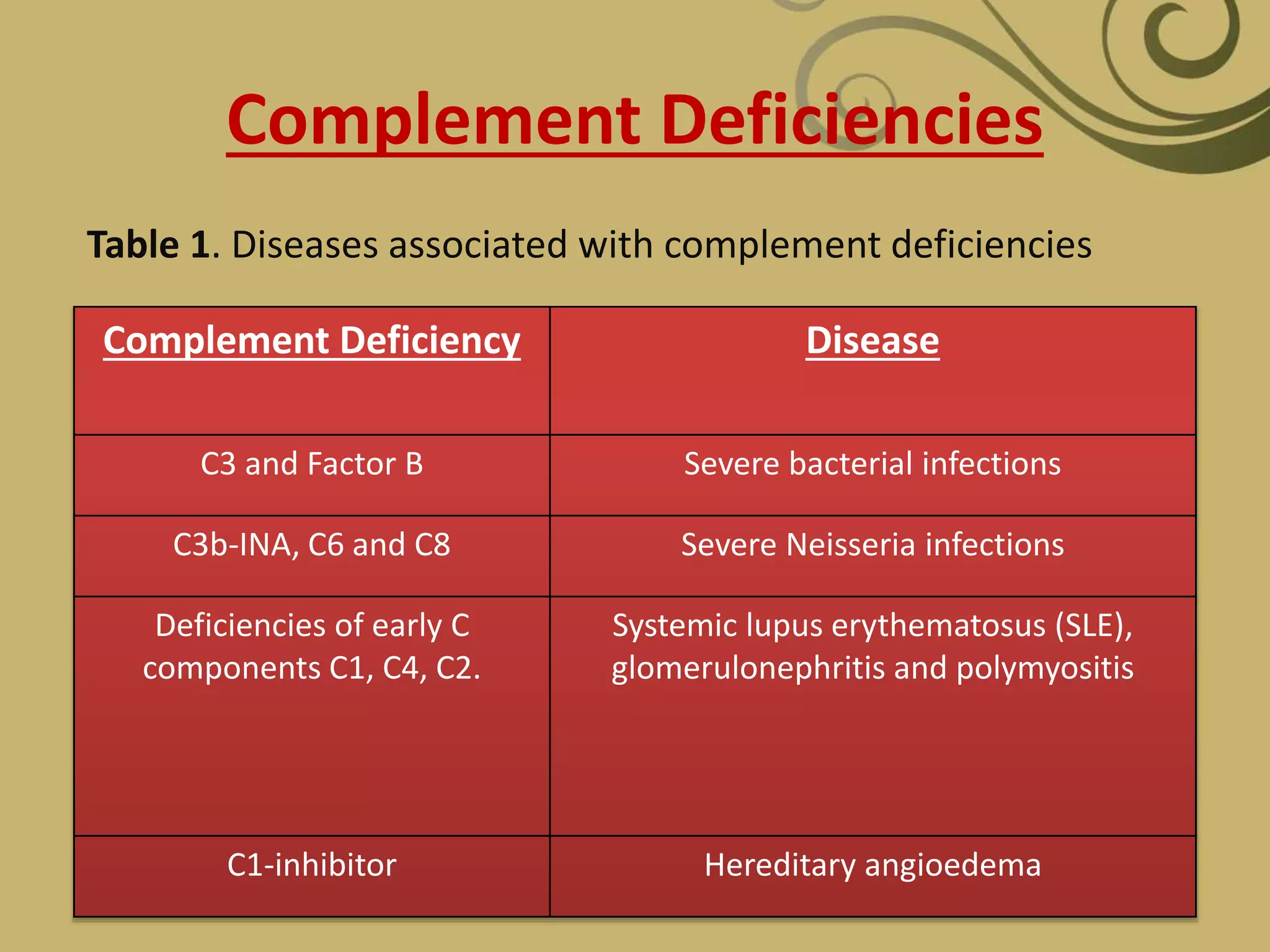
The complement system is part of the innate immune system and consists of over 30 proteins. It was originally identified in the 1890s by Jules Bordet and Paul Ehrlich as a heat-labile component of serum that enhanced the ability of antibodies to kill bacteria. There are three complement activation pathways: the classical pathway which is initiated by antibody-antigen complexes, the lectin pathway which is activated by mannose-binding lectin, and the alternative pathway which is spontaneously activated by microbial surfaces. Complement activation results in opsonization, inflammation, and formation of the membrane attack complex to kill microbes. Deficiencies in specific complement components can increase susceptibility to certain infections.