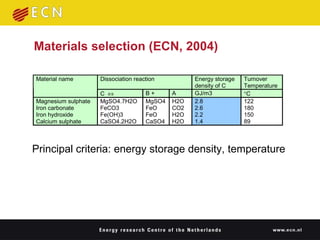
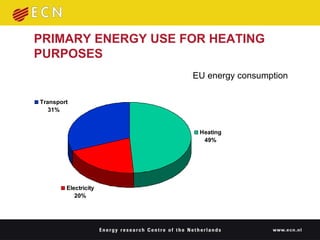
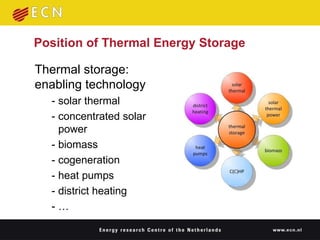
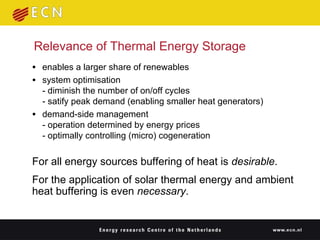
1. Thermal energy storage (TES) technologies like phase change materials (PCMs), sorption, and thermochemical materials can store solar and renewable heat for use when needed.
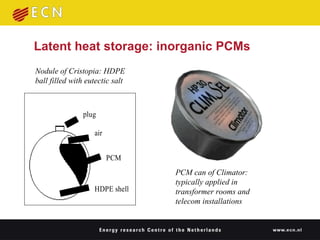
2. PCMs use the heat of phase change during melting and freezing to efficiently store and release thermal energy. Organic PCMs like paraffin wax are promising due to their high storage density and melting temperatures around human comfort levels.
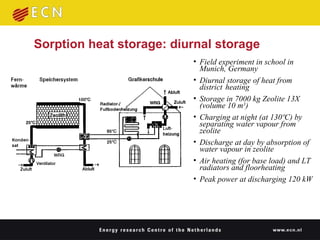
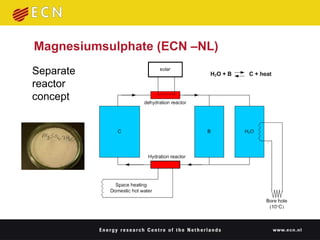
3. Sorption technologies use physical or chemical bonding to store heat in materials like silica gels, zeolites, or chemical reactions. A demonstration used zeolite to store nighttime heat from district heating for use during the day.




![Characteristics of TES Values Unit temperature level [ºC] specific energy density [kJ/kg] or [MJ/m 3 ] thermal power [kW] Categories Choice between - time to market research/test,demo/available for market storage period day / week, month / season Building integration possible / not possible](https://image.slidesharecdn.com/20090123ltstorppt-1232721166810387-1/85/Compact-Thermal-Energy-Storage-9-320.jpg)