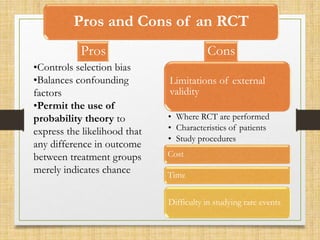
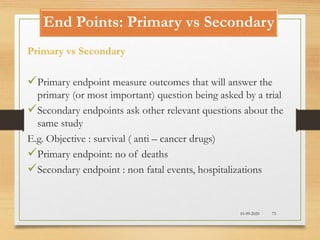
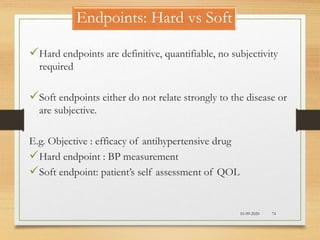
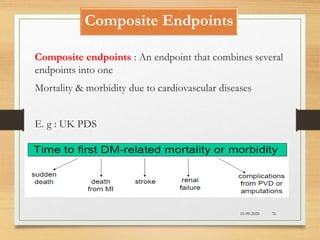
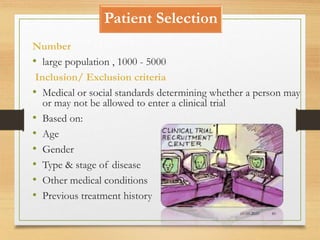
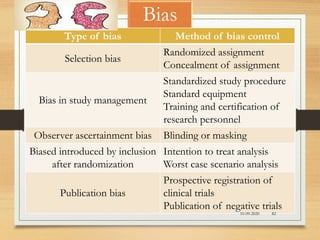
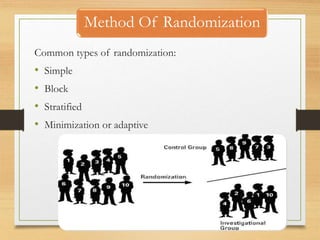
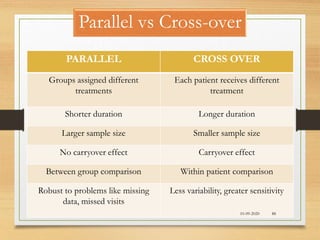
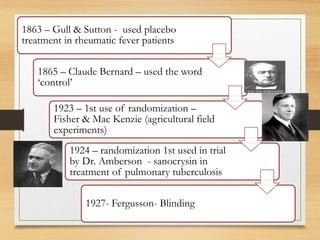
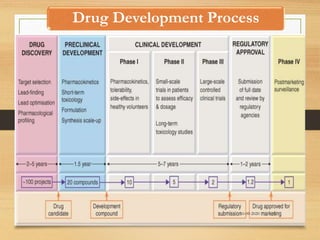
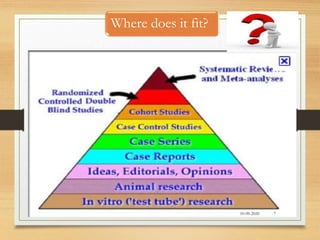
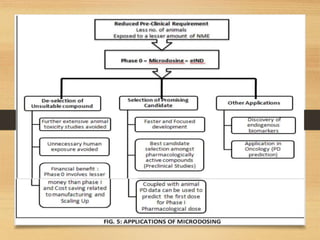
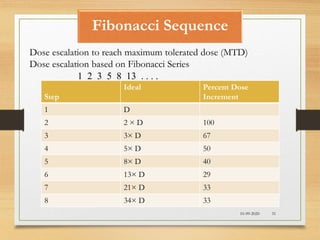
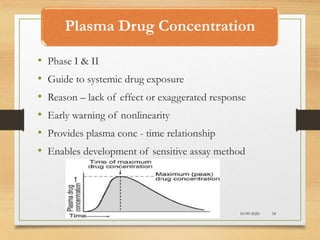
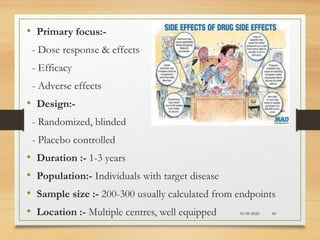
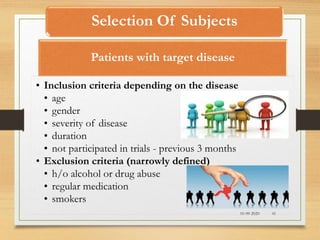
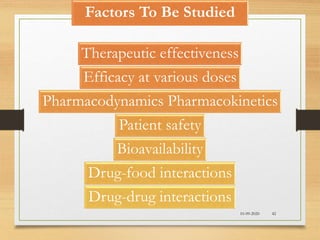
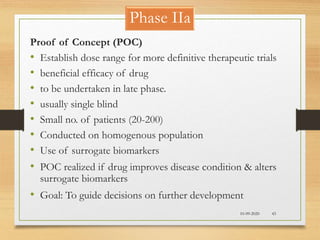
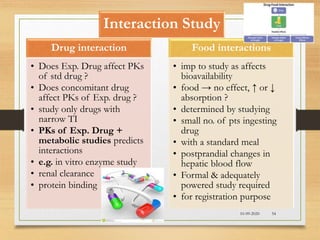
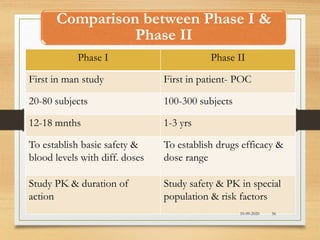
The document provides a comprehensive overview of clinical trial phases, highlighting historical developments, definitions, and methodologies relevant to drug development from phase 0 to phase III. It emphasizes the significance of micro-dosing in early trials, the importance of safety and tolerability studies, and the design elements crucial for effective evaluations of new therapies. Regulatory and ethical considerations, alongside the financial impacts of various phases, are also thoroughly discussed.































![10-09-2020 61
Informed Consent
• Communication between researcher and participant
• Nuremberg Code [4], the Declaration of Helsinki [5], the
Belmont Report [3], and the International Ethical
Guidelines for Biomedical Research Involving Human
Subjects.
• Surrogate consent i/c/o emotionally/ mentally impaired and
pediatric population.](https://image.slidesharecdn.com/clinicaltrialsphases0-3-200910085919/85/Clinical-trials-phases-0-3-54-320.jpg)