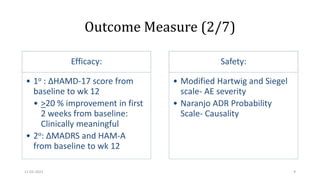
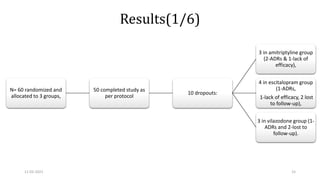
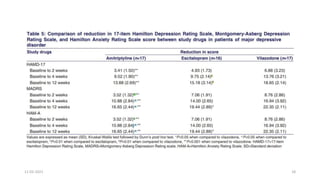
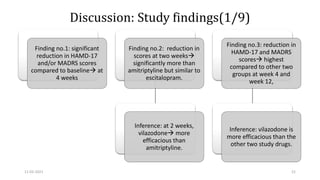
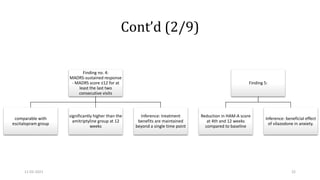
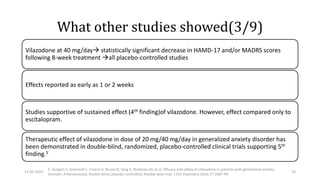
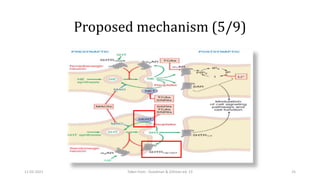
The document presents a critical appraisal of a journal article on vilazodone for treating major depressive disorder (MDD) and generalized anxiety disorder (GAD). It summarizes a study comparing vilazodone to escitalopram and amitriptyline, highlighting vilazodone's greater efficacy and tolerability, though noting the study's small sample size and limitations. The authors suggest vilazodone as a treatment option for select MDD patients with anxiety, pending further research.













![Evaluation of Safety Profile (6/9)
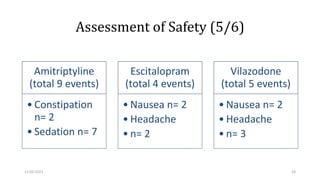
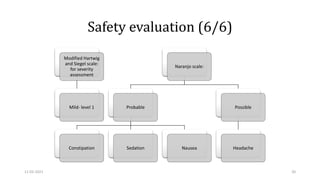
Adverse effects with antidepressant drugs are common
negatively impact patient outcomes.
vilazodone (29.41%) and escitalopram (25%) groups comparable
amitriptyline group (52.94%) significantly higher
Intolerability to medication is one of the most common reasons of discontinuation of antidepressant
treatment.[29]
Constipation and sedation were major adverse events
11-02-2021 26](https://image.slidesharecdn.com/forslideshare-critical-appraisalofarticle-210211073045/85/For-slideshare-How-to-Critically-Appraise-a-Journal-Article-26-320.jpg)
![Cont’d (7/9)
Nausea and headache major adverse events in escitalopram and vilazodone
groups.
GI s/e of mild severity and transient.
Sexual dysfunction is a common adverse effect of SSRIs which are currently the
most commonly used antidepressants.[4]
Sexual dysfunction as a s/e reported by none.
11-02-2021 27](https://image.slidesharecdn.com/forslideshare-critical-appraisalofarticle-210211073045/85/For-slideshare-How-to-Critically-Appraise-a-Journal-Article-27-320.jpg)