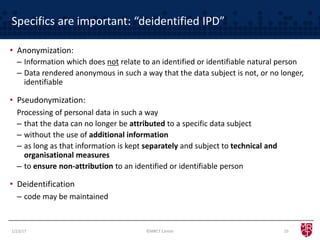
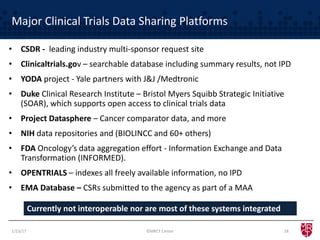
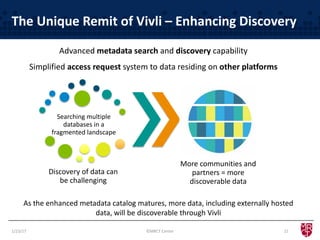
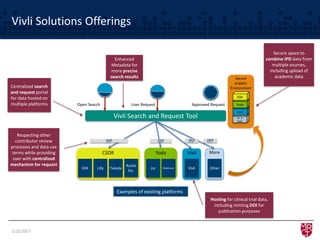
The document discusses the evolving landscape of clinical trial data sharing, highlighting changes in transparency mandates and the rise of data sharing requirements by government agencies. It outlines the legal frameworks in the EU and US regarding data sharing, emphasizes the importance of utilizing research data, and identifies gaps in current data-sharing practices and platforms. The text also introduces Vivli as a centralized solution to facilitate data sharing and access across various repositories.