Embed presentation
Download to read offline






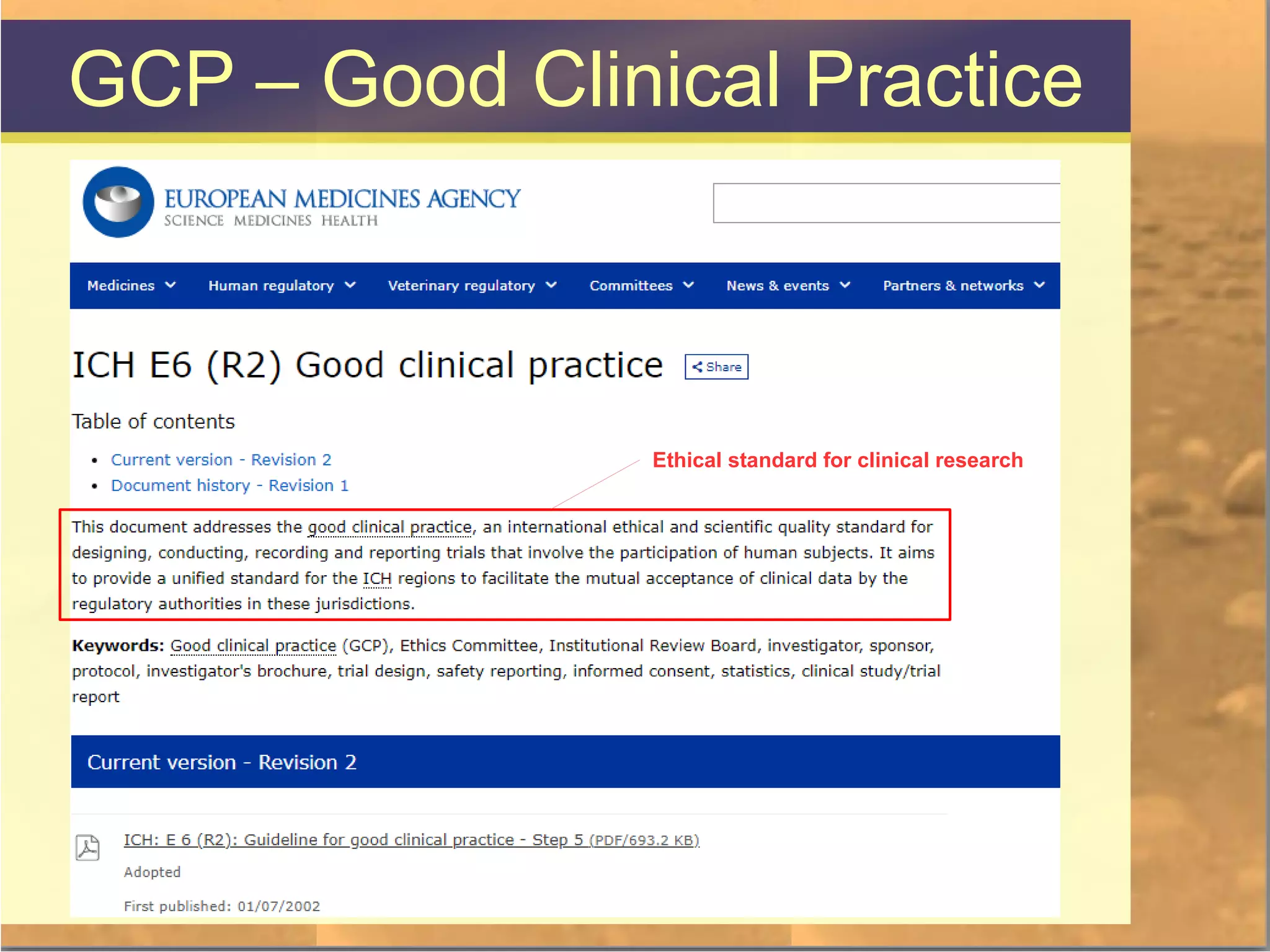





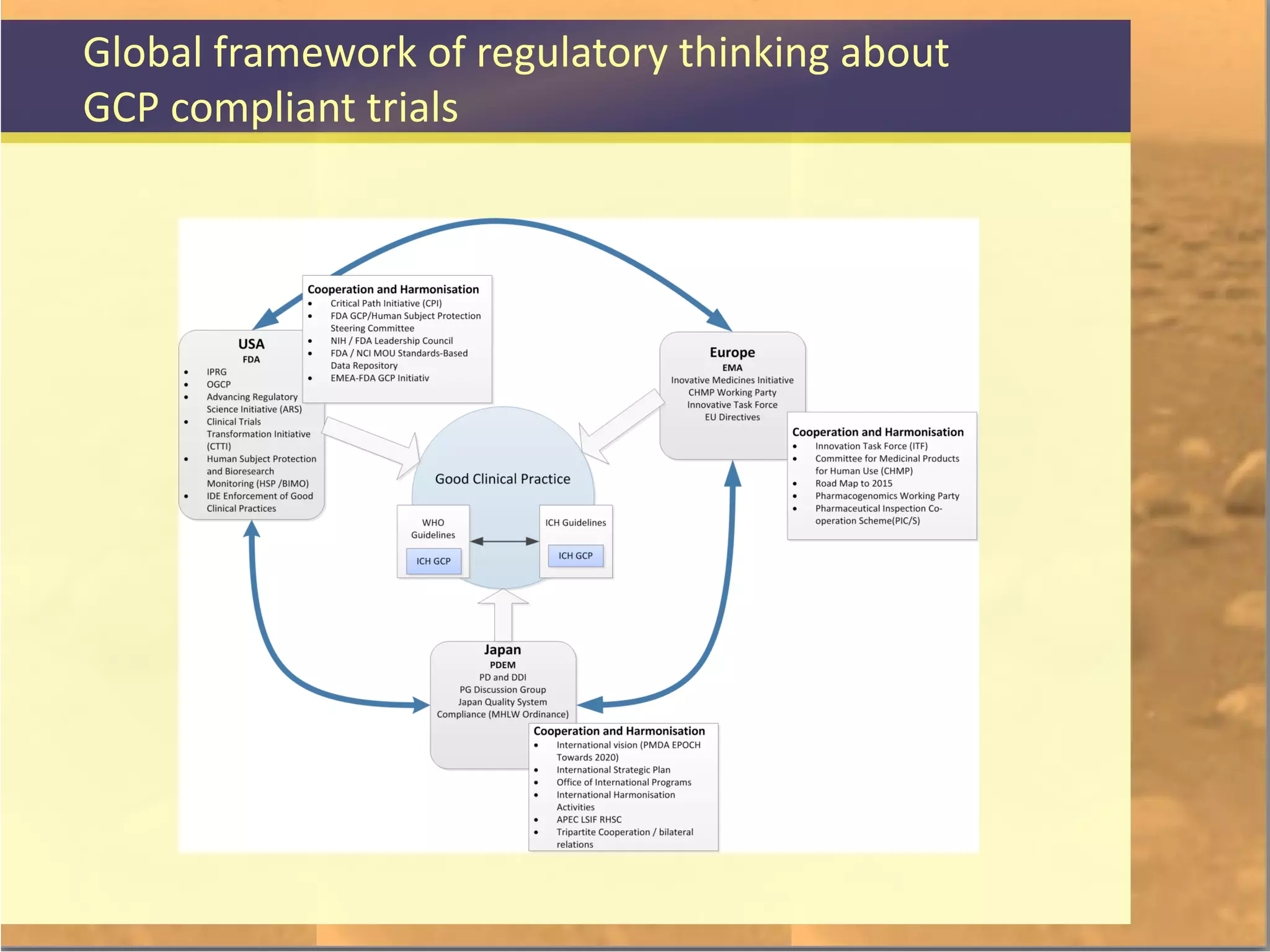



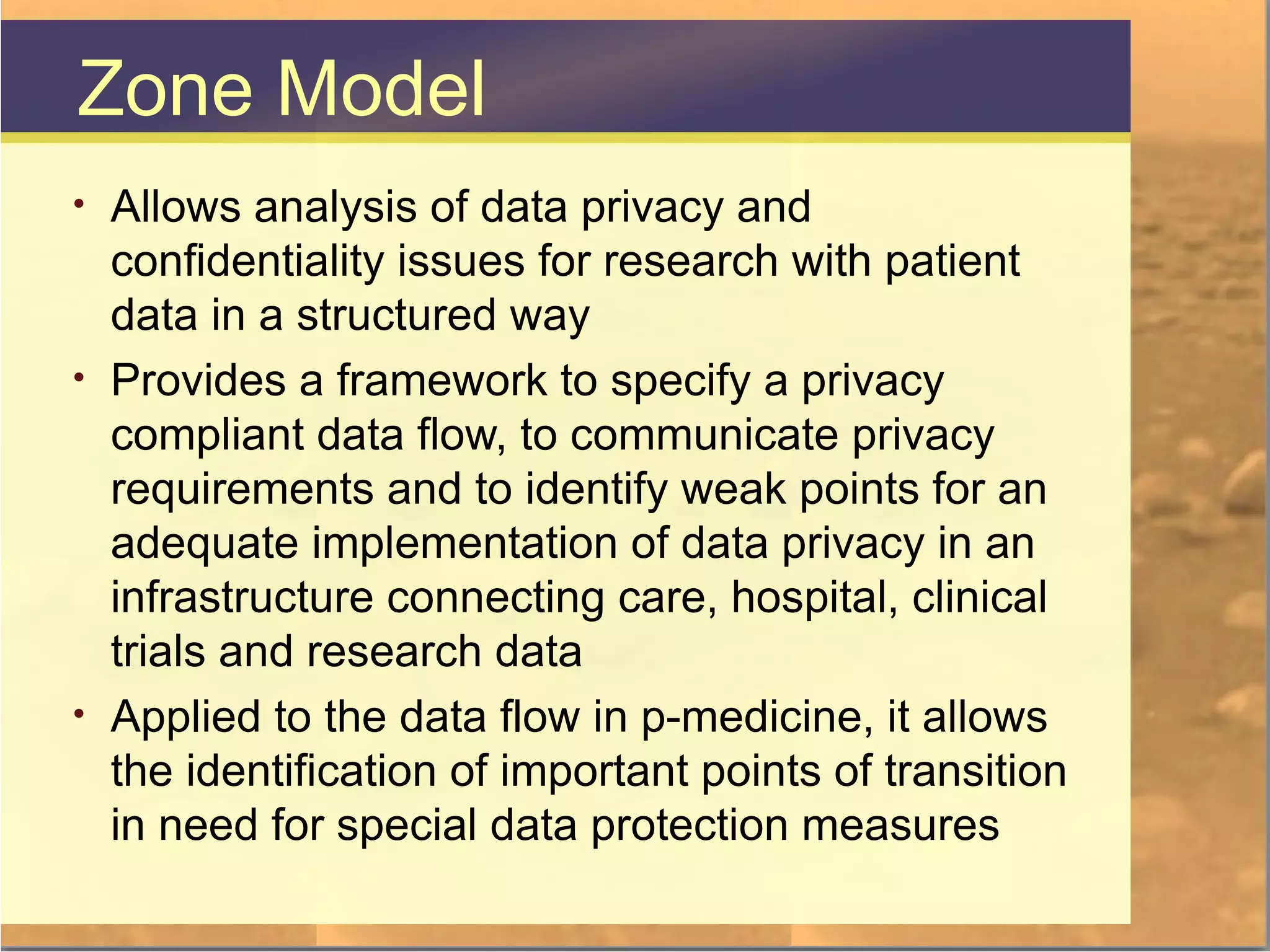
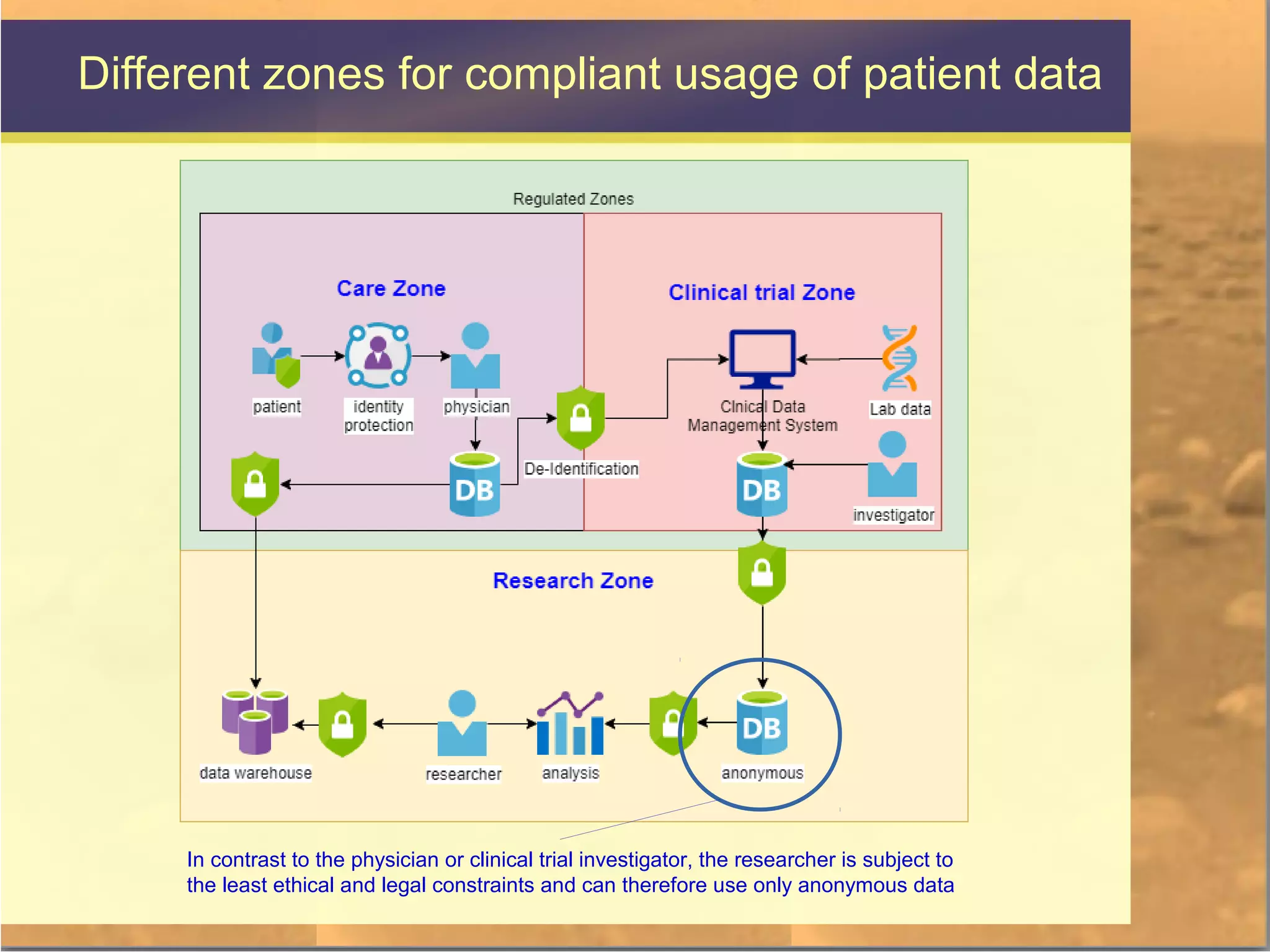



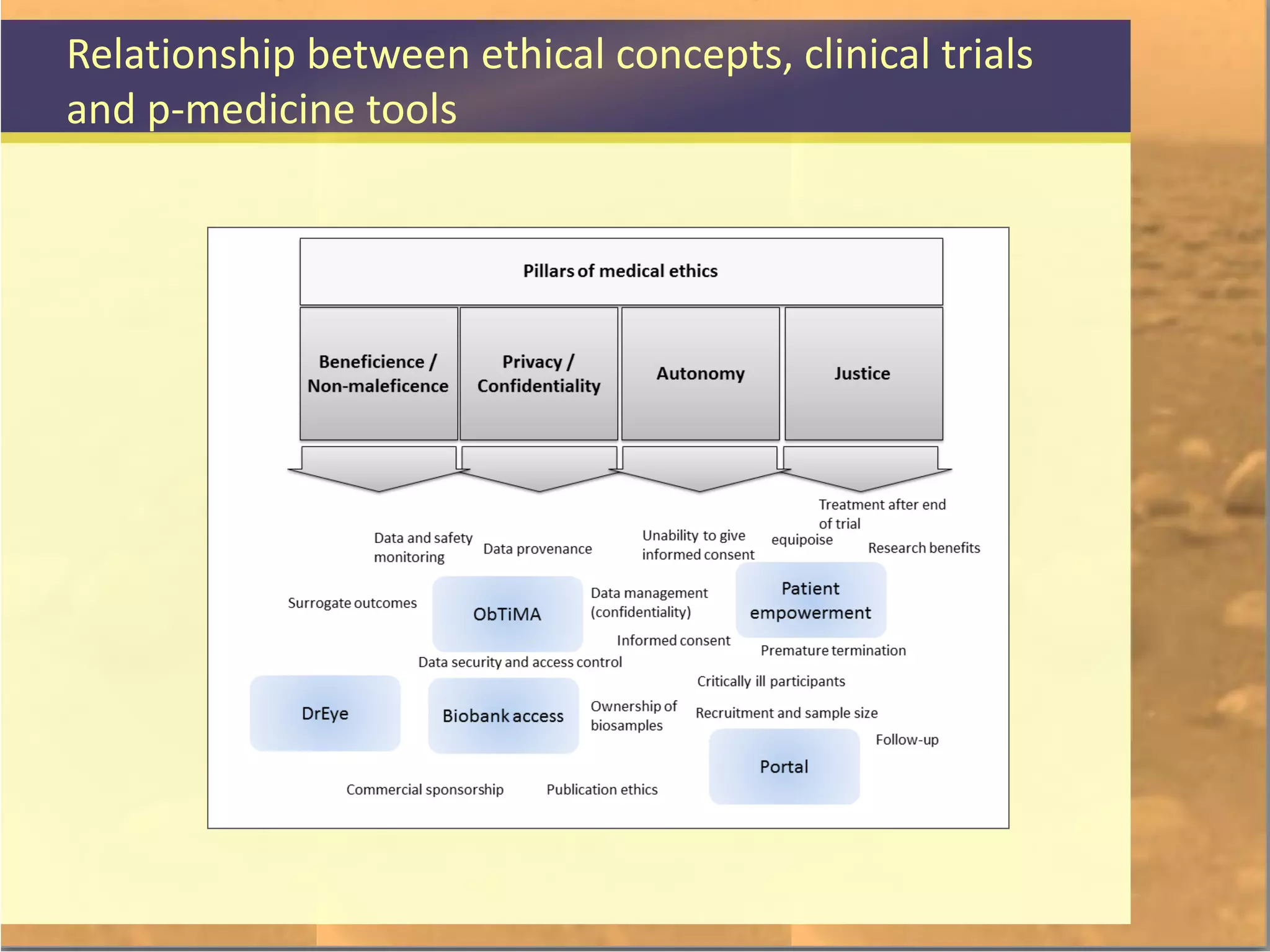


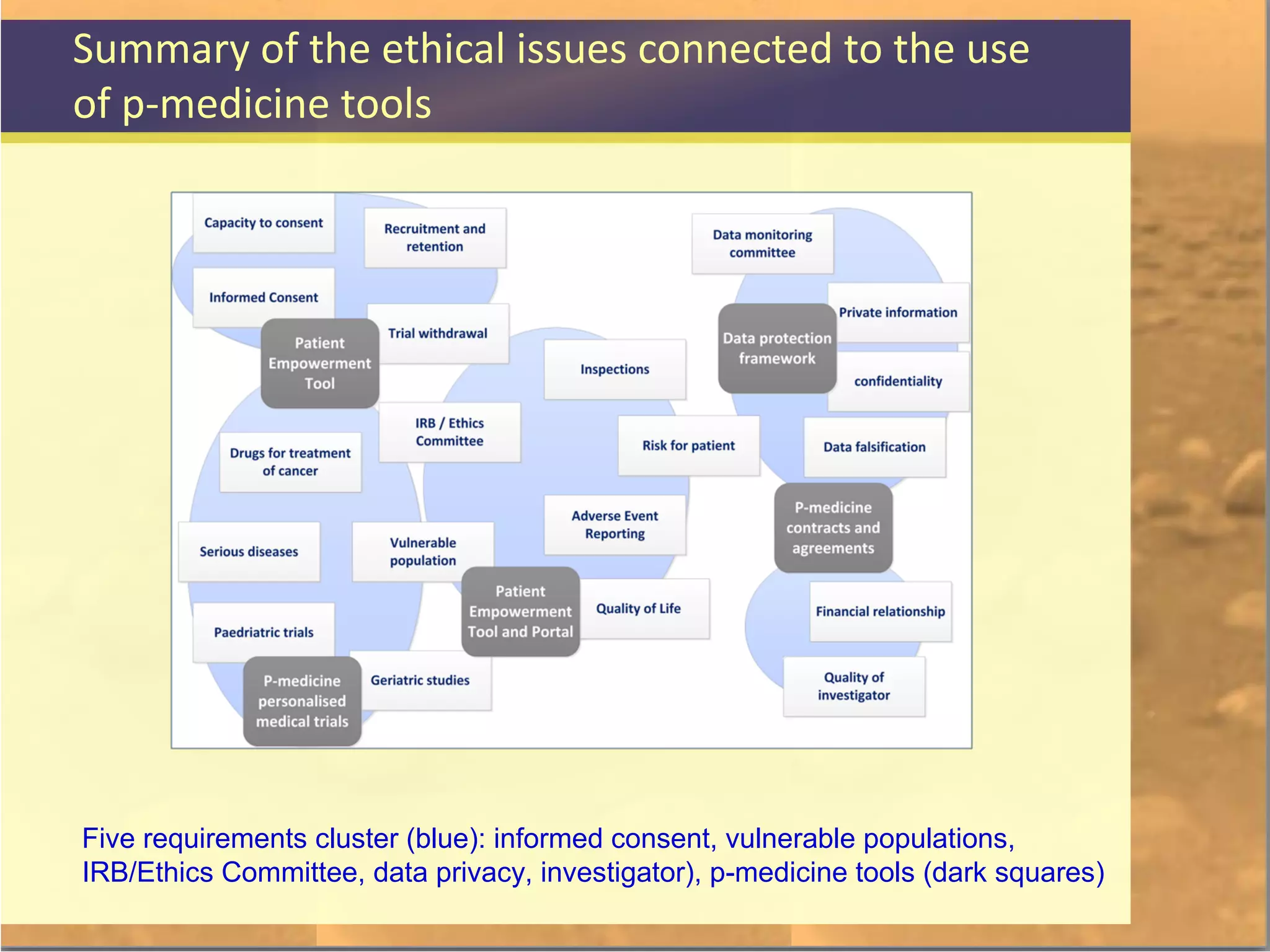
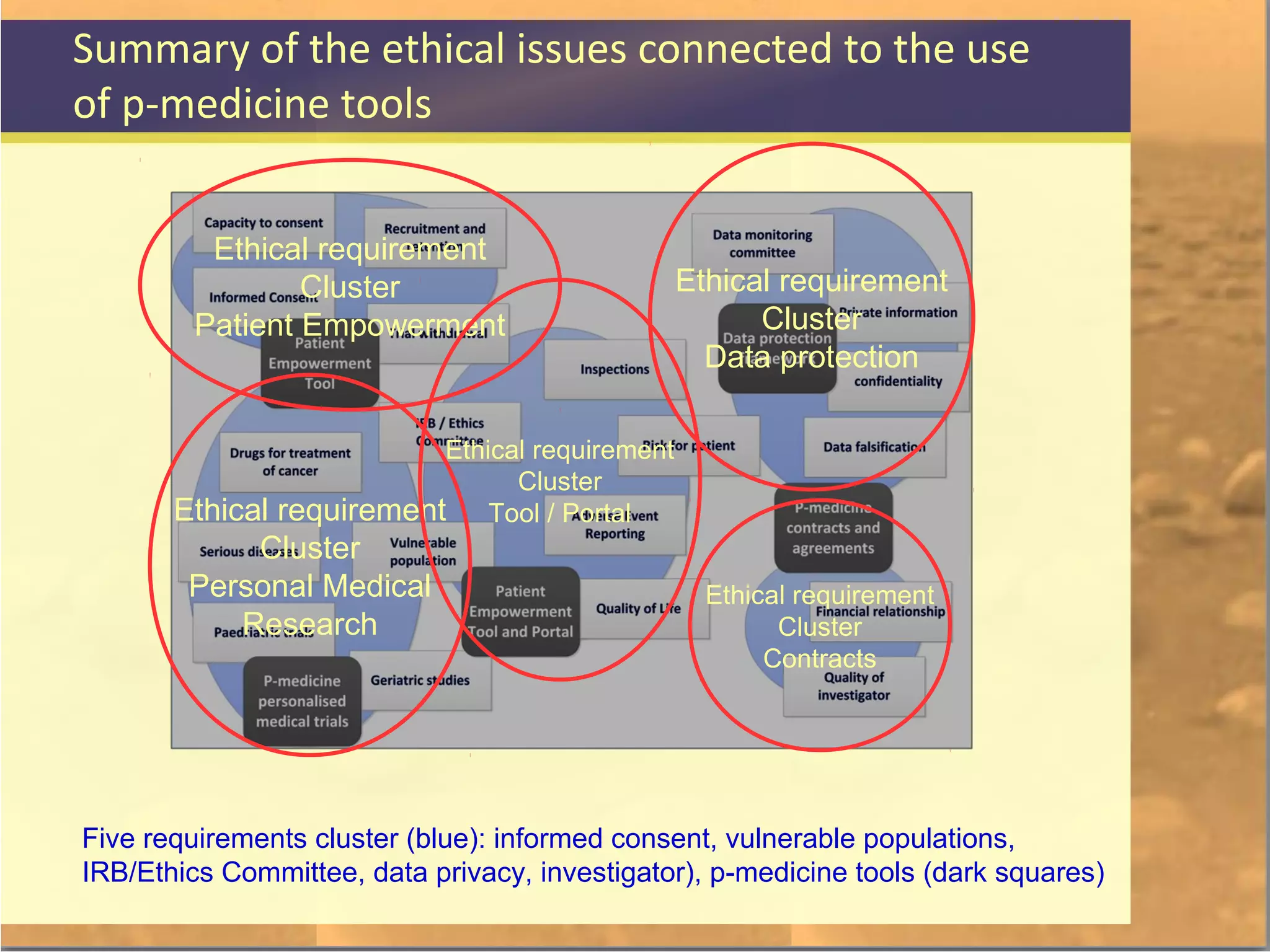
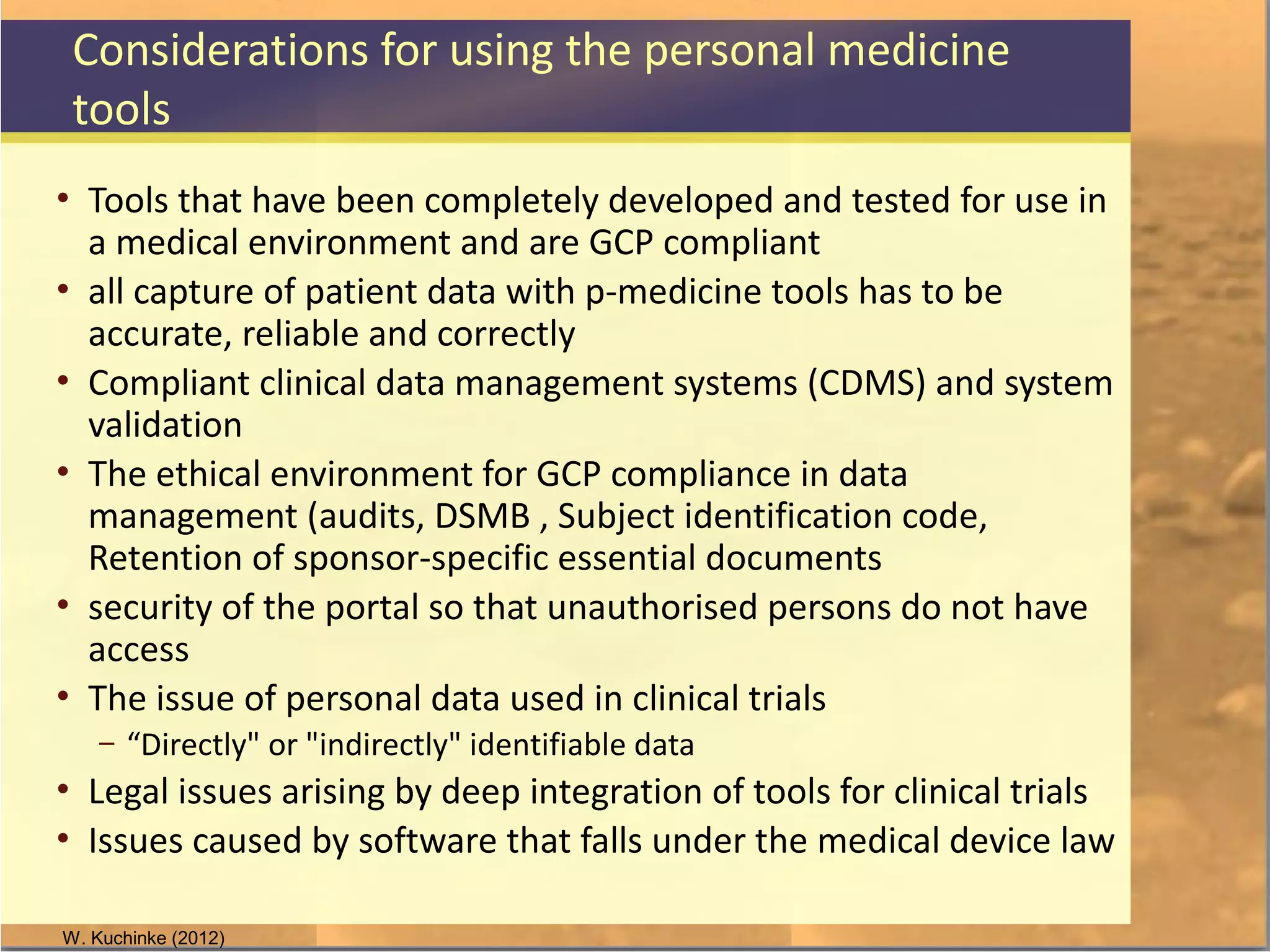
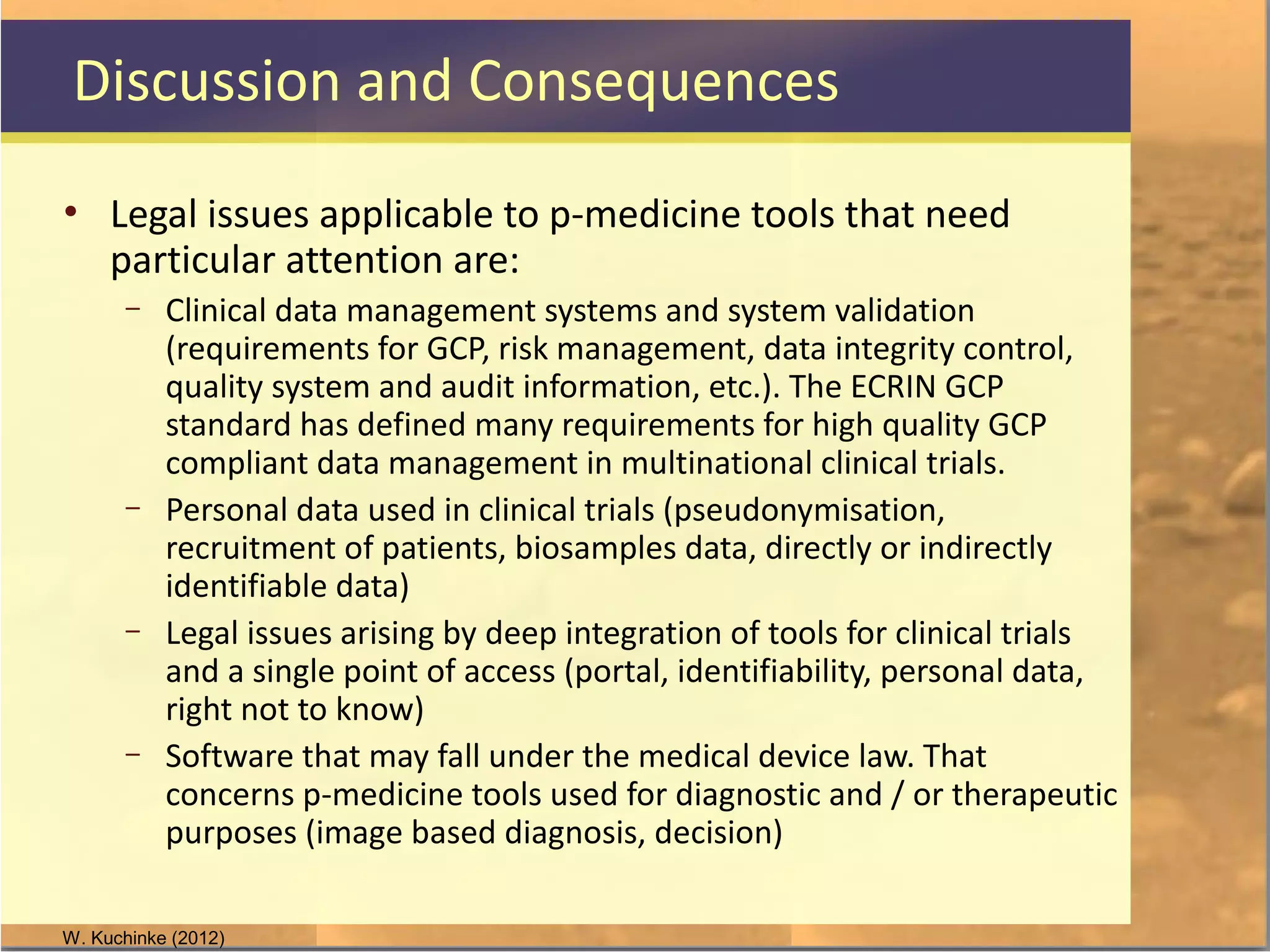
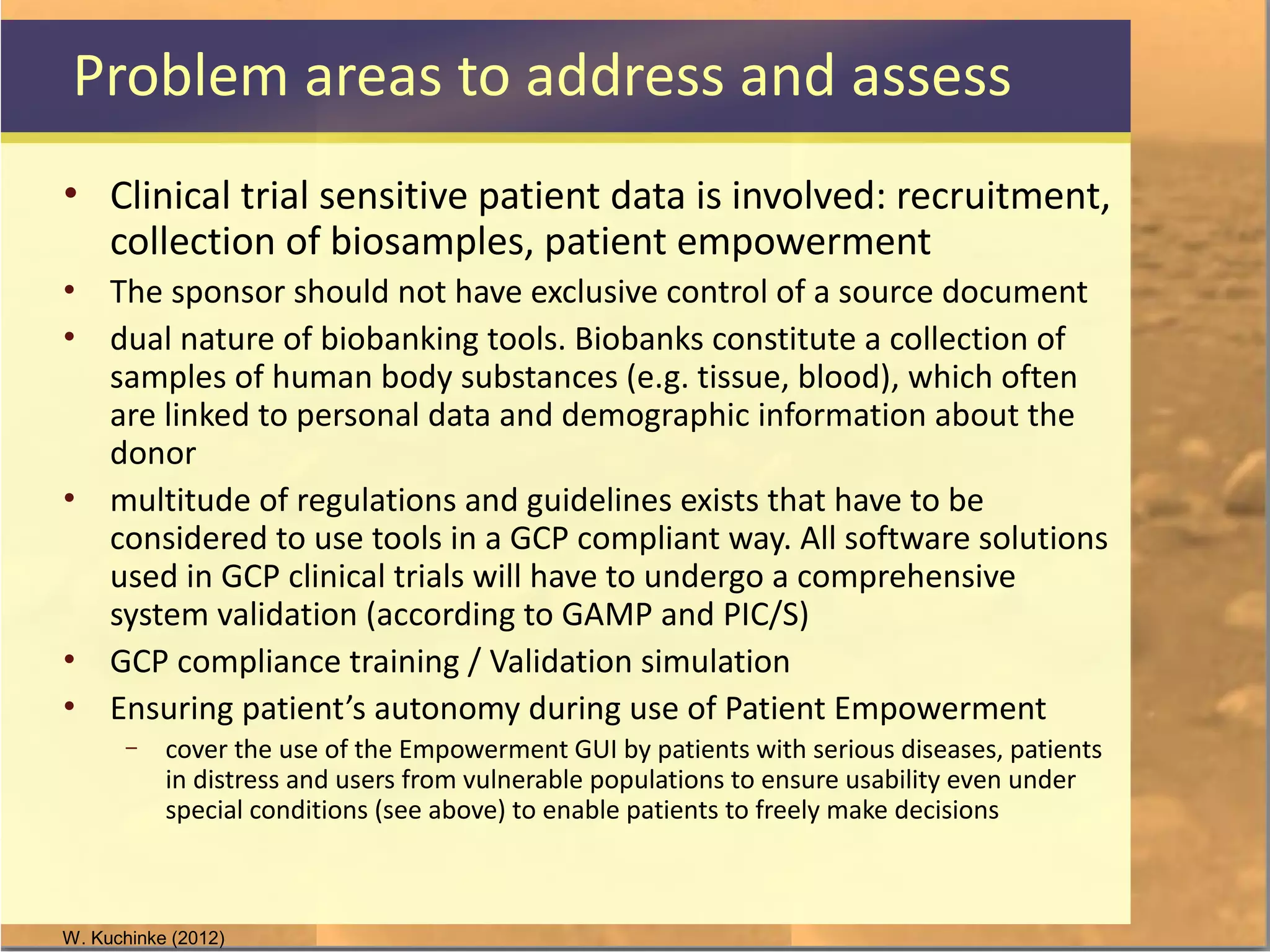


The document discusses legal and ethical issues related to international clinical trials, particularly within the context of p-medicine. It emphasizes the importance of Good Clinical Practice (GCP) guidelines, which ensure the ethical conduct and safety of clinical trials, addressing issues like informed consent, data privacy, and compliance with regulations. Additionally, it highlights challenges surrounding data management and the utilization of software tools in clinical trials, stressing the need for rigorous ethical standards and legal compliance.































