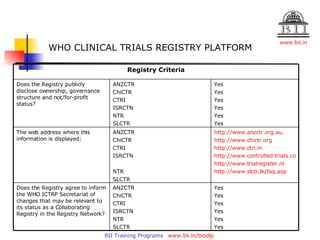
The document discusses clinical trials registries and the Clinical Trials Registry-India (CTRI). It describes how CTRI was established to increase transparency and accessibility of clinical trial data in India. It provides details on CTRI's mission and dataset, requirements for trial registration, and its role as a primary registry that collects and shares data according to WHO guidelines.


































![THANK YOU For Further Details & Enquiries, Contact: Bioinformatics Institute of India C-56A / 28, Sector – 62 Noida – 2010301, U.P Tel: 0120-4320801/02 Mobile: 9818473366, 9810535368 Email: [email_address] Visit: www.bii.in](https://image.slidesharecdn.com/clinical-trials-registry-1214556708183172-8/85/Clinical-Trials-Registry-36-320.jpg)