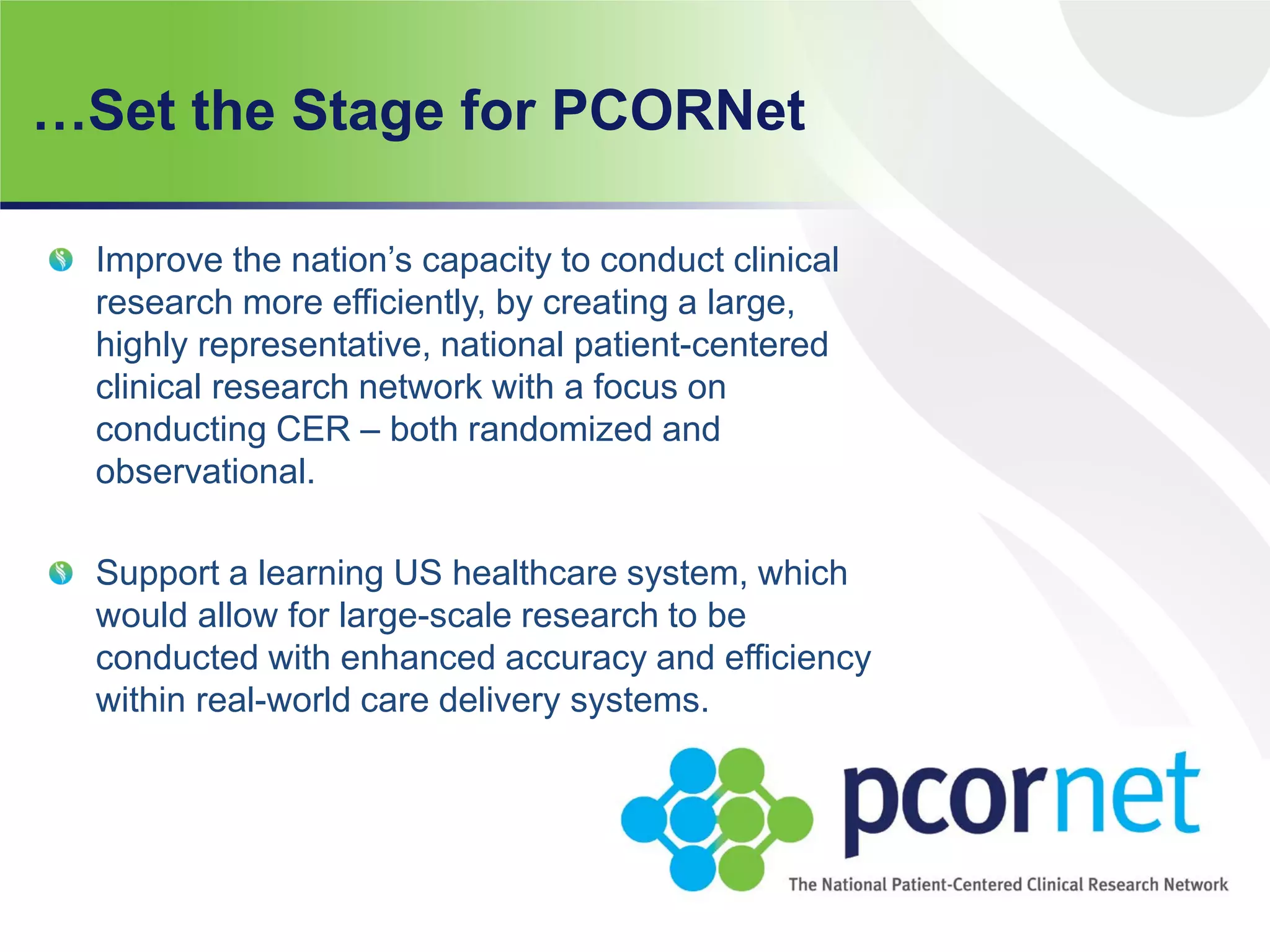
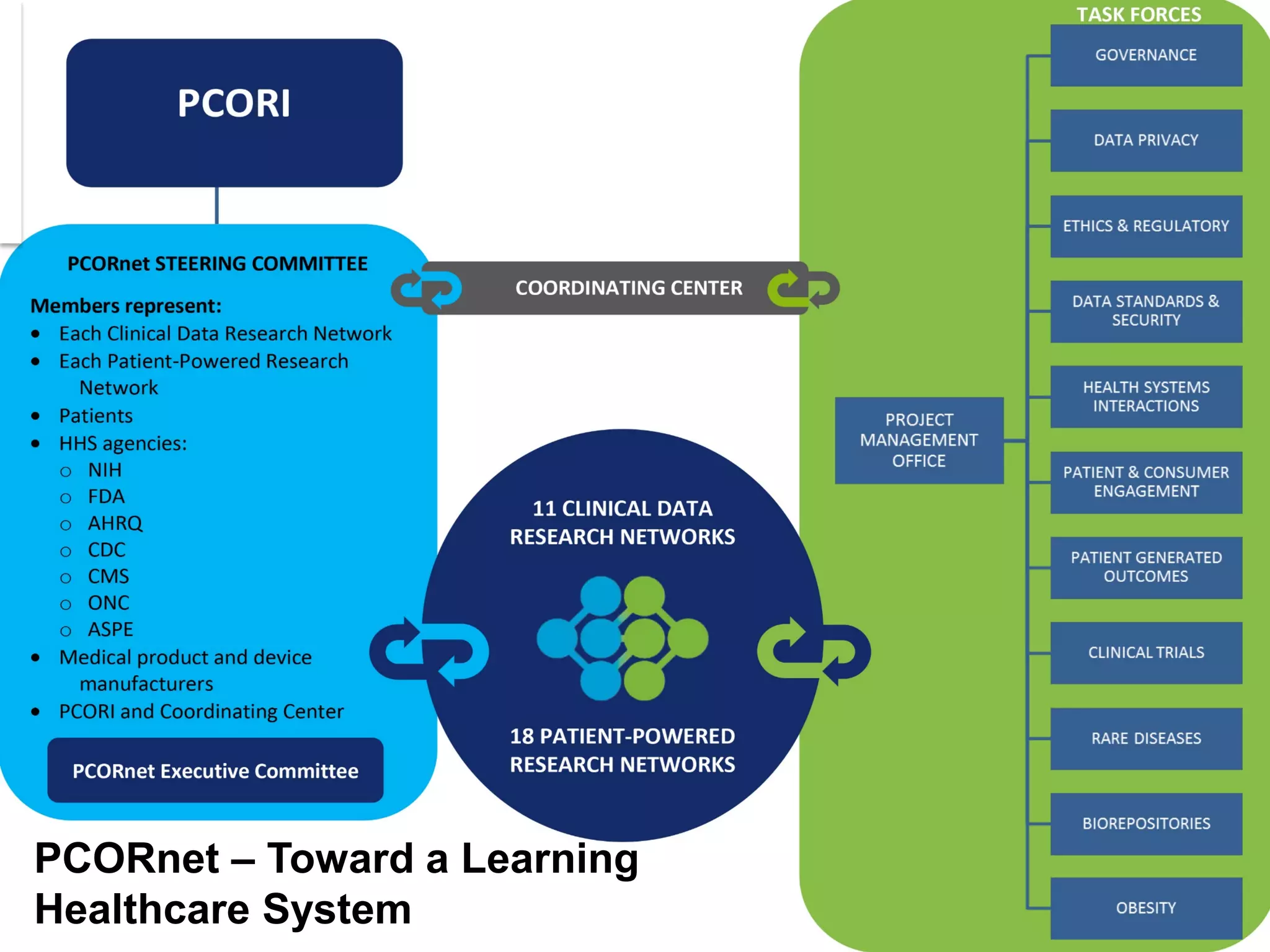
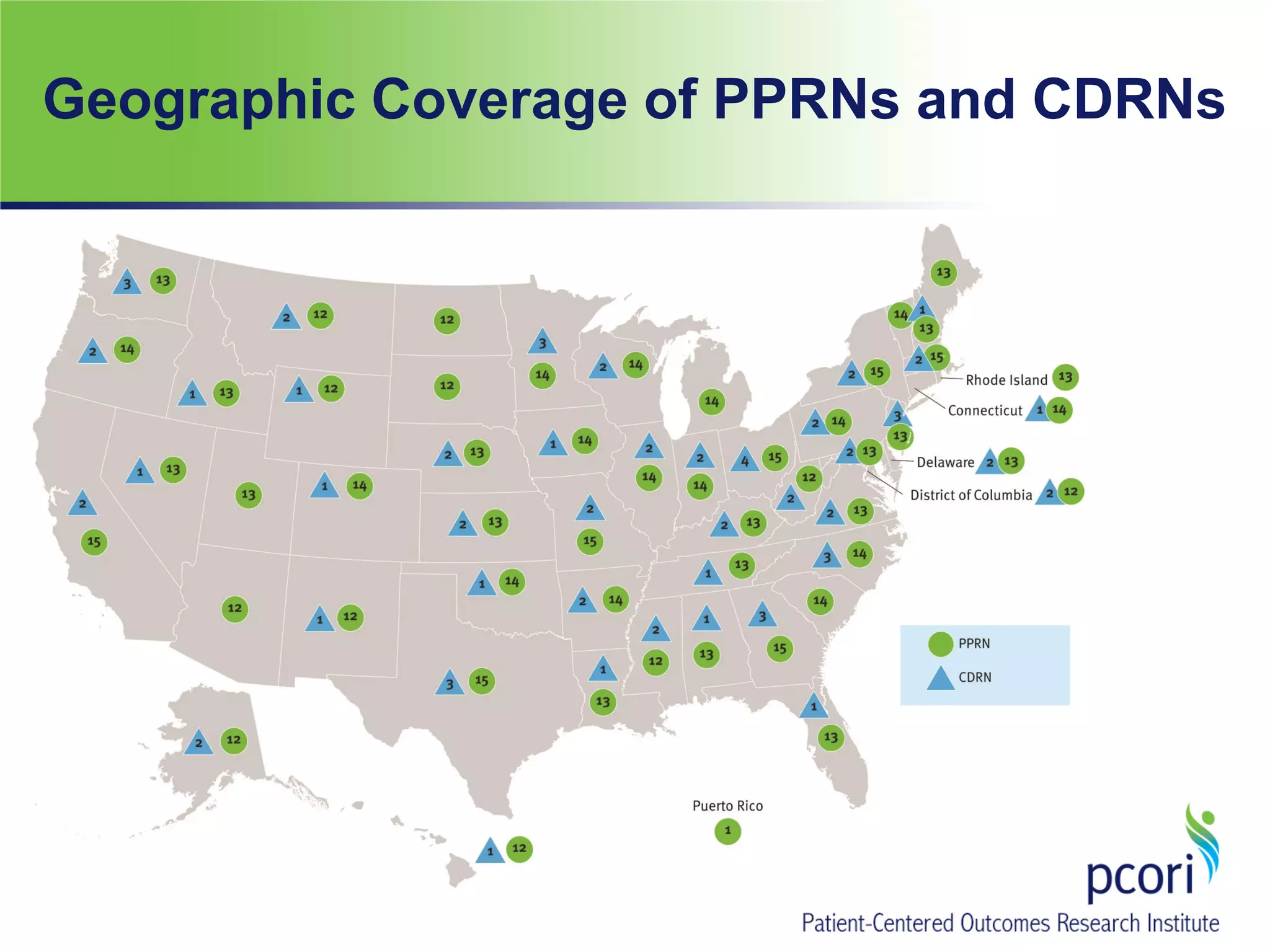
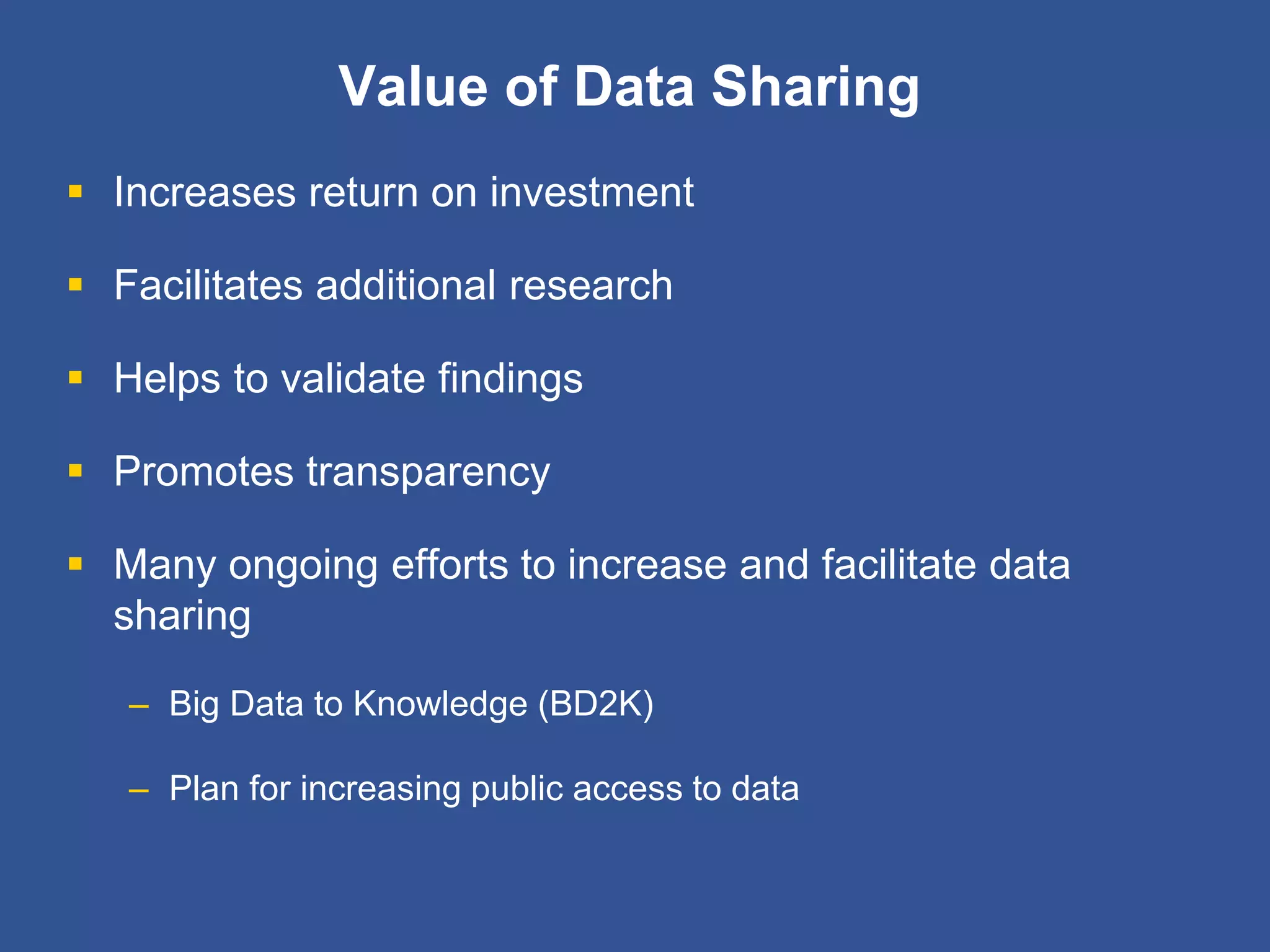
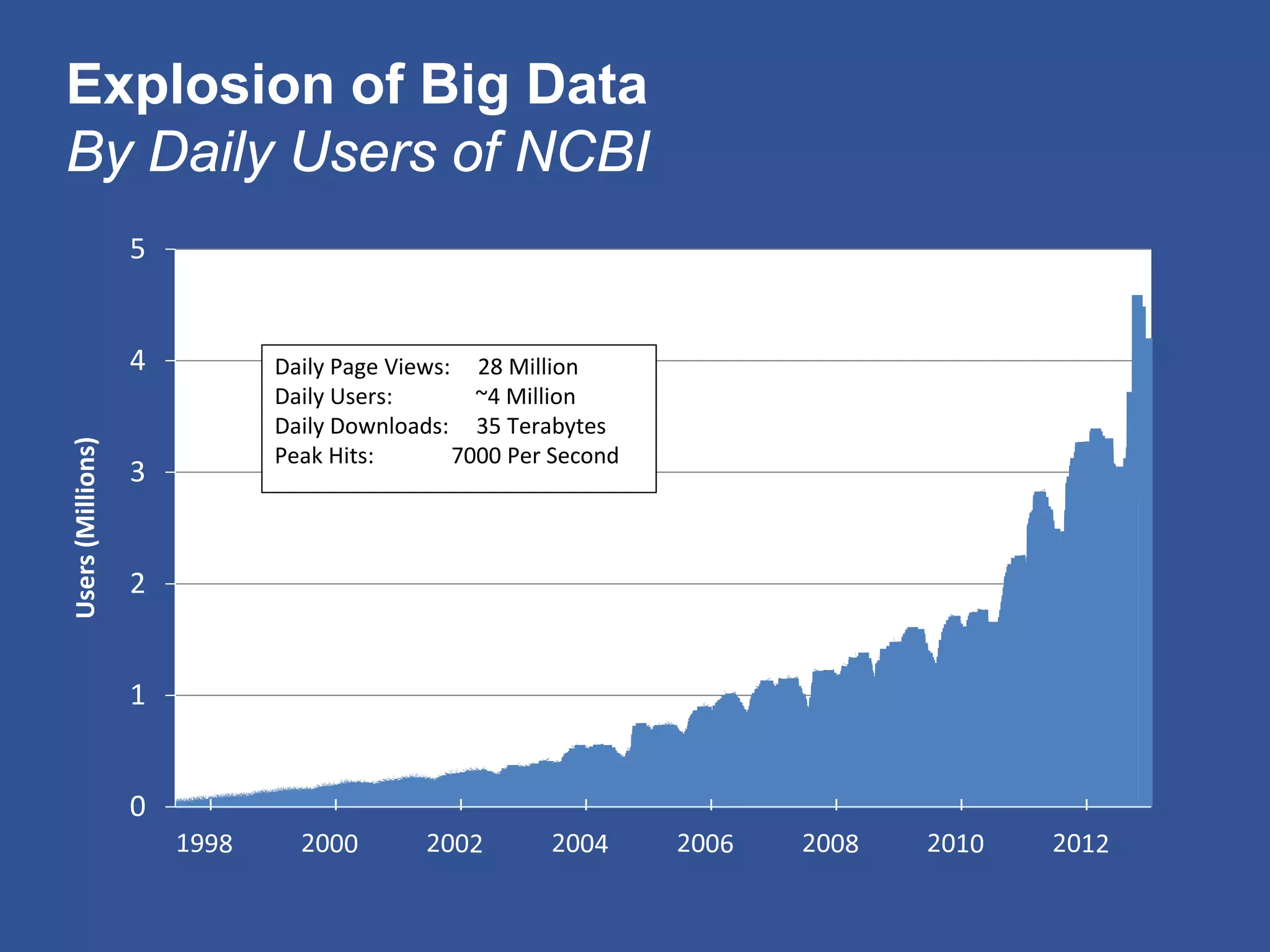
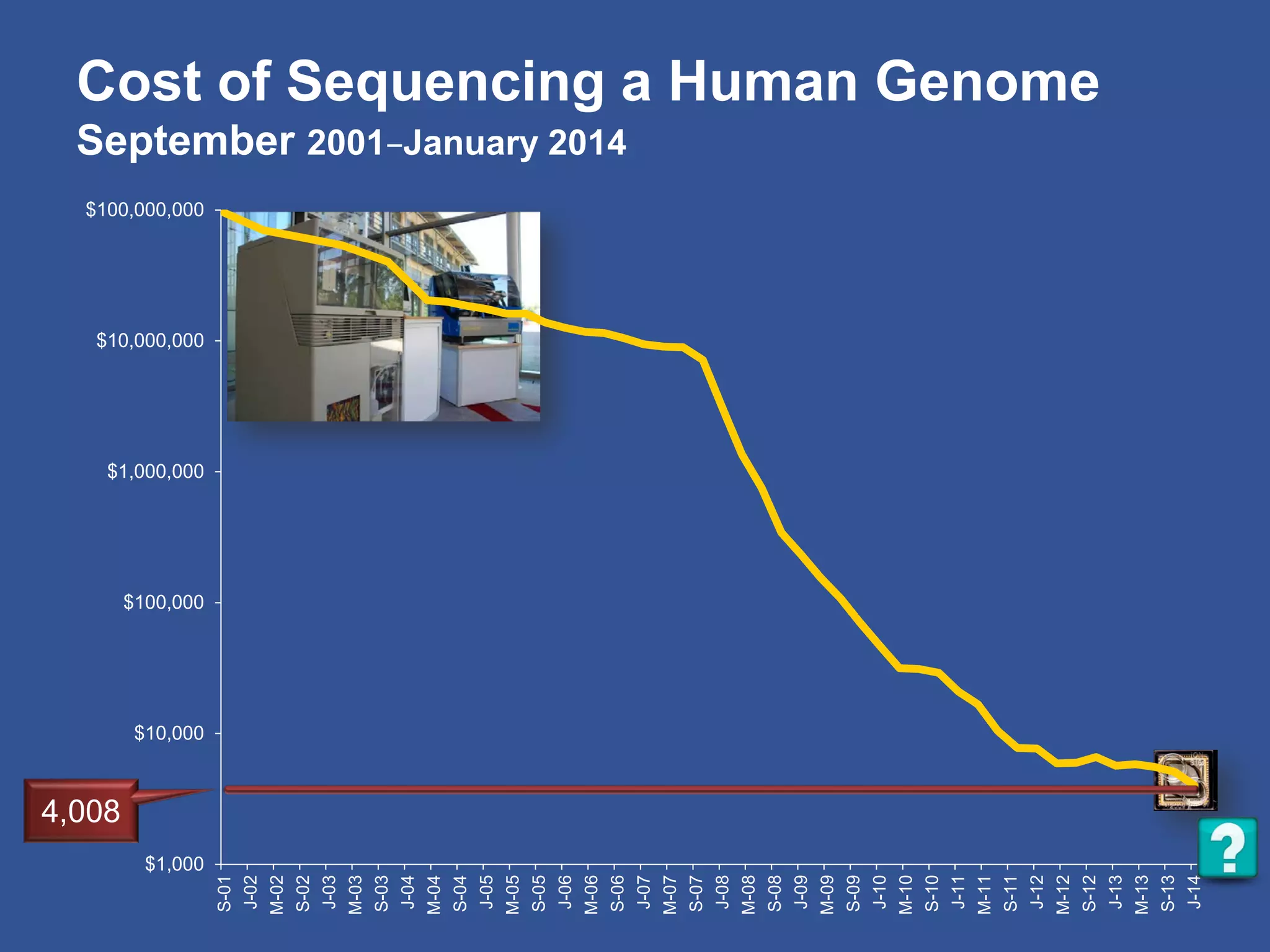
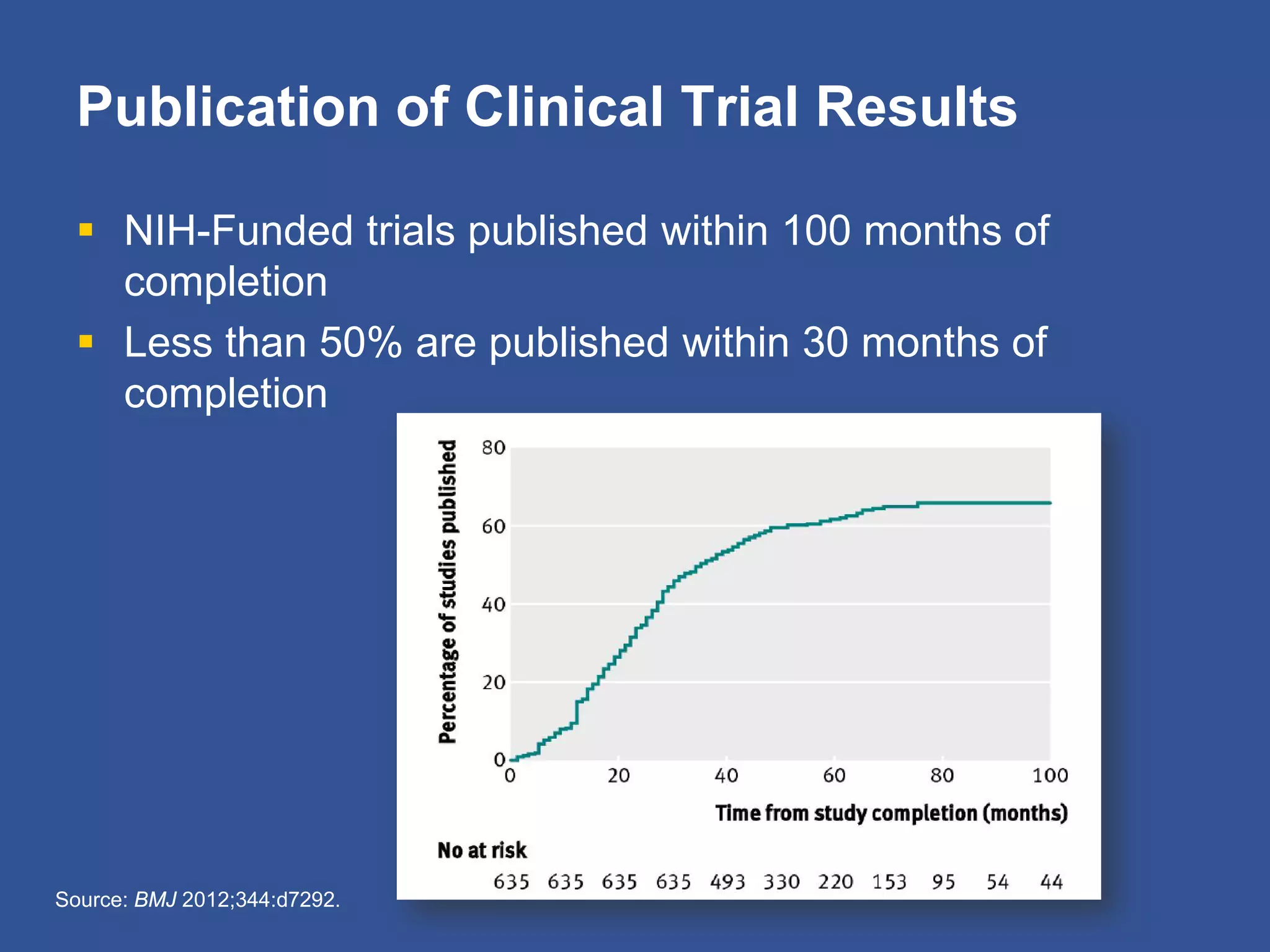
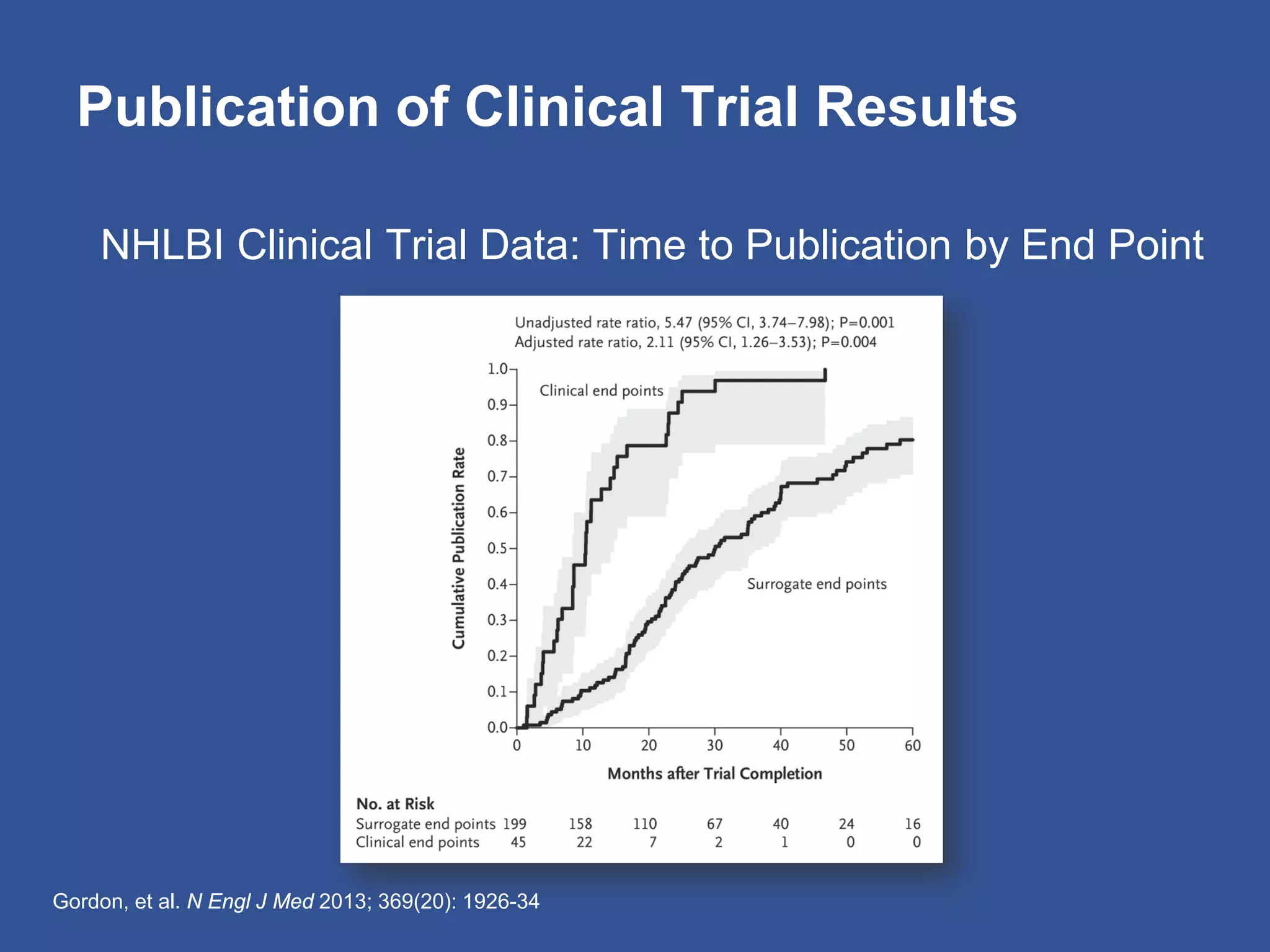
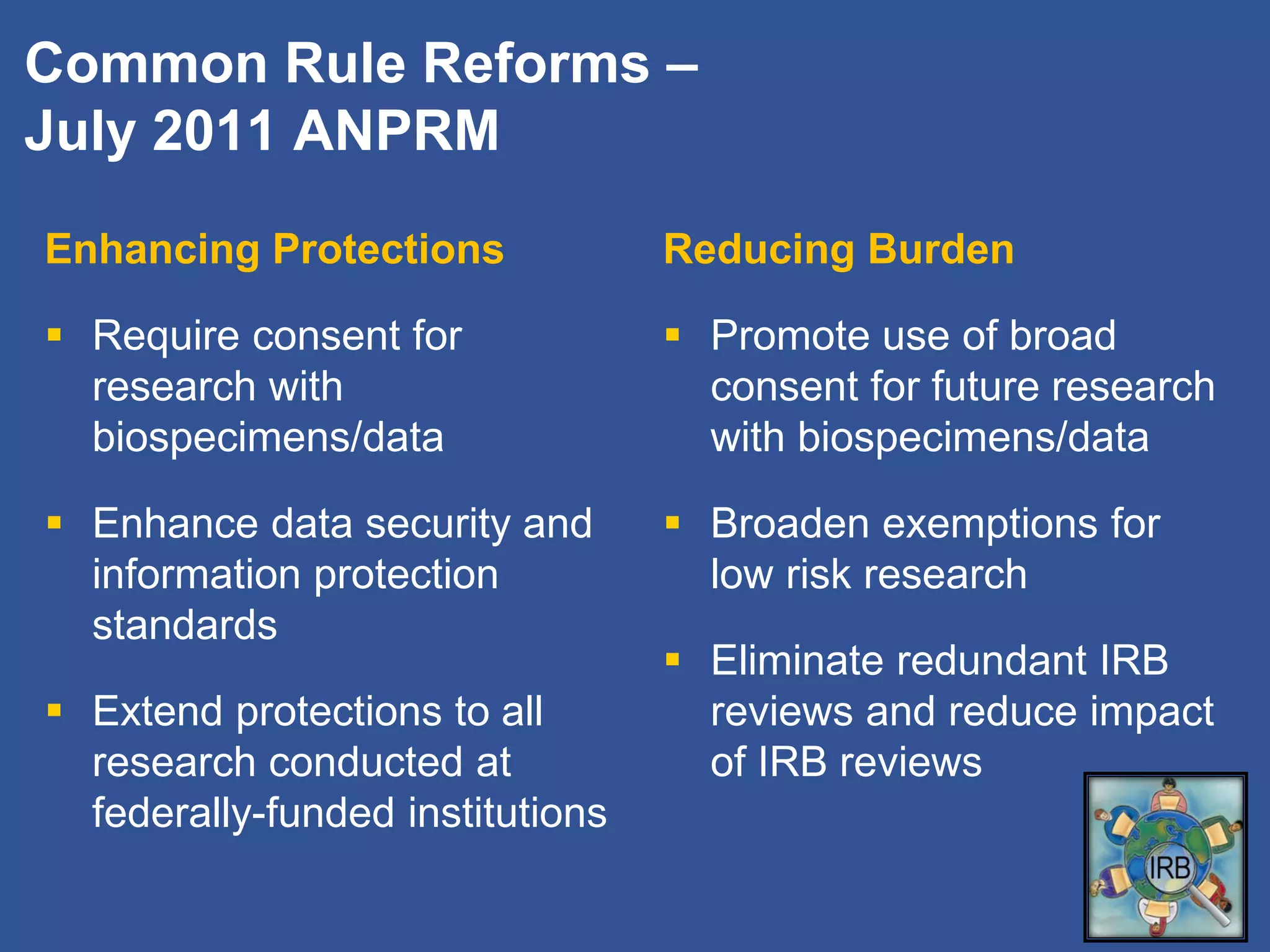
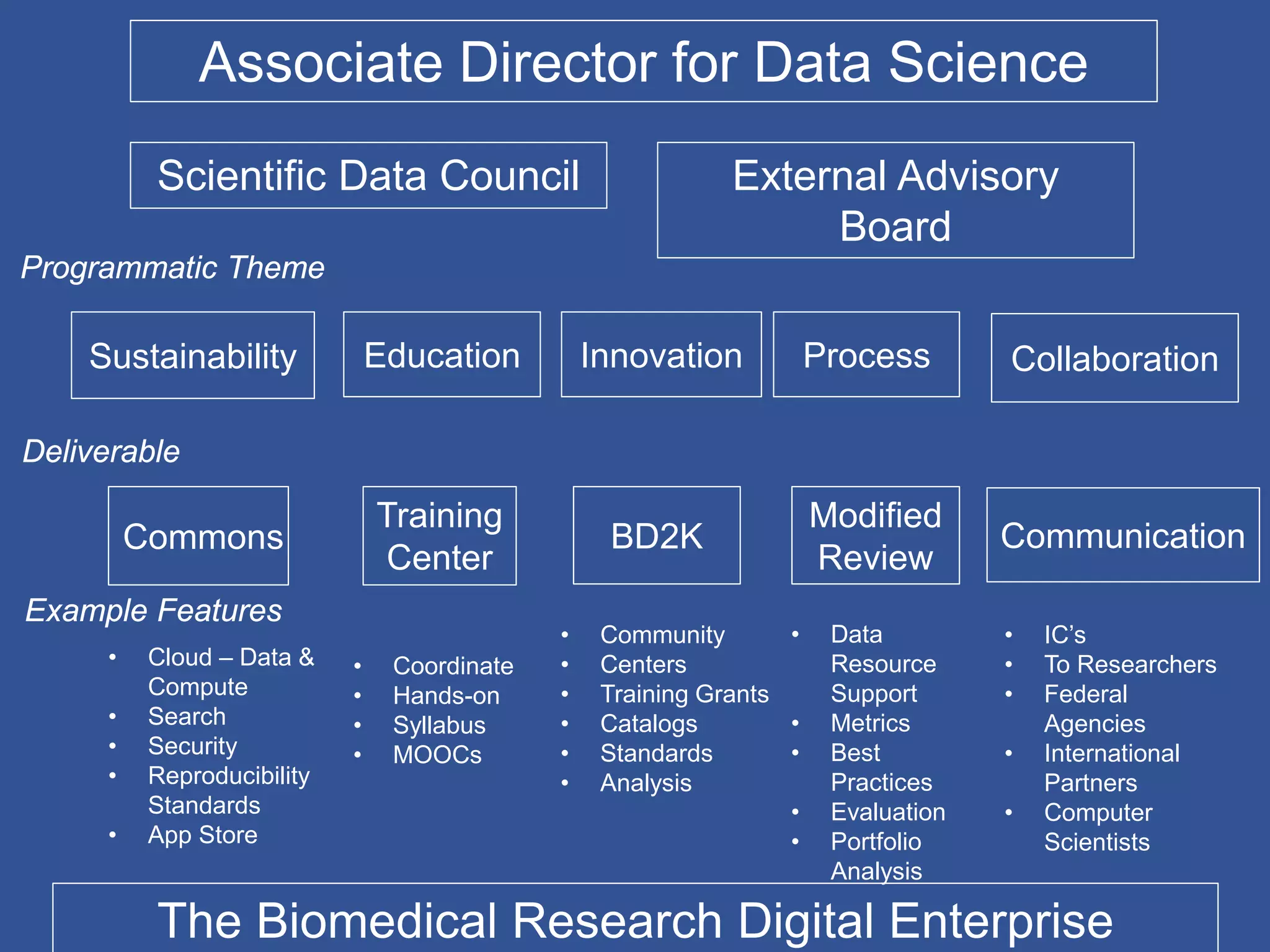
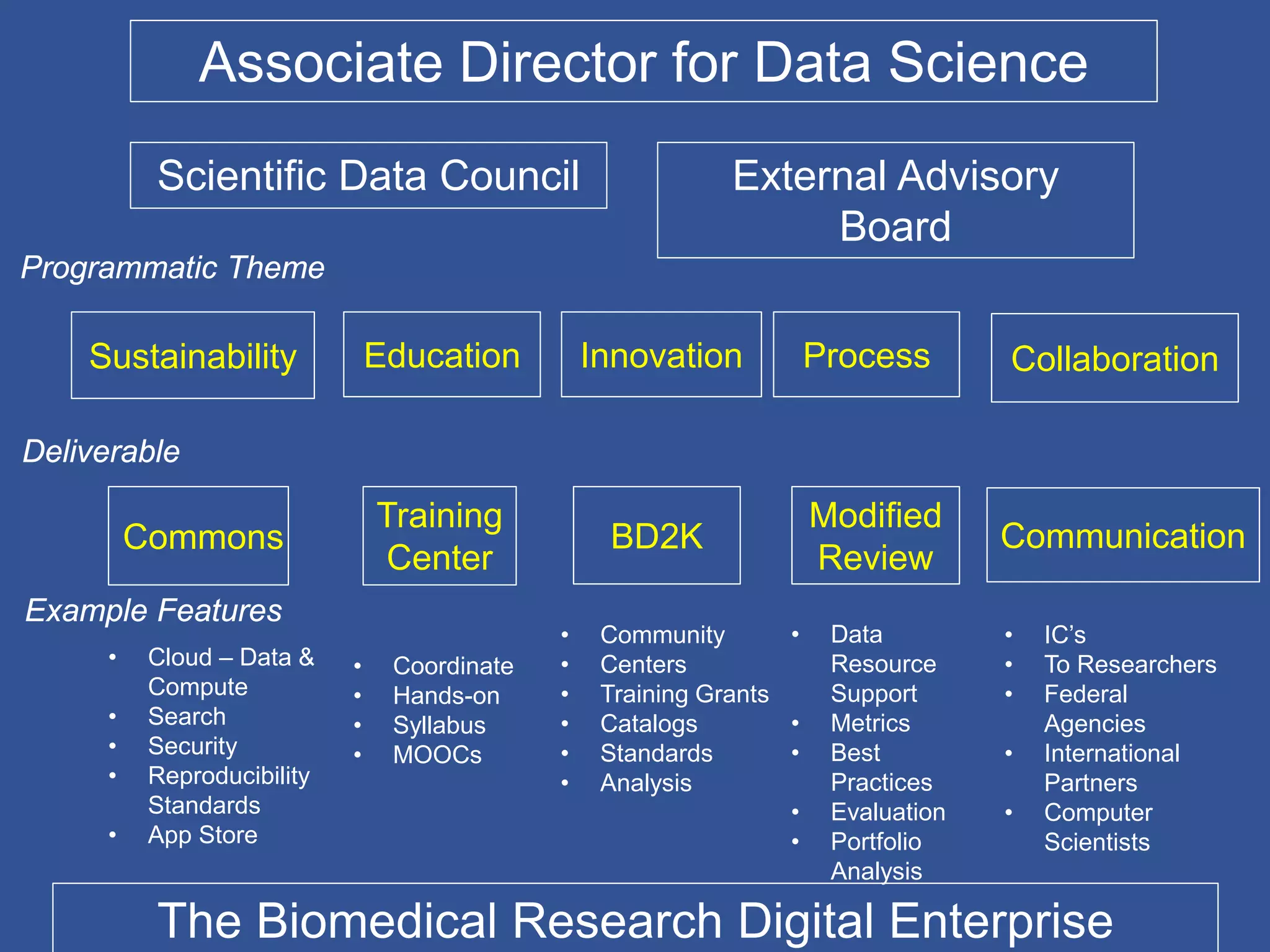
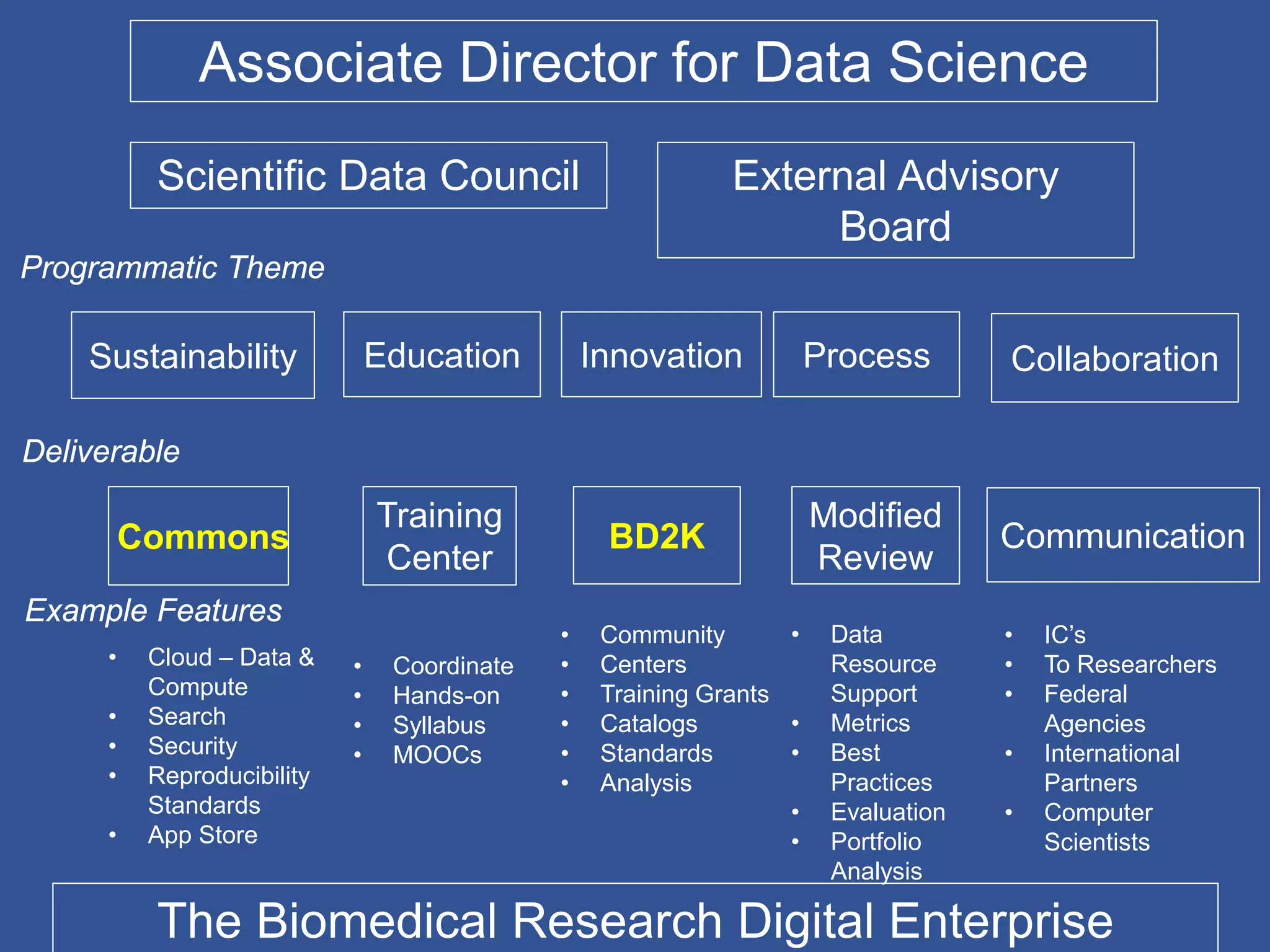
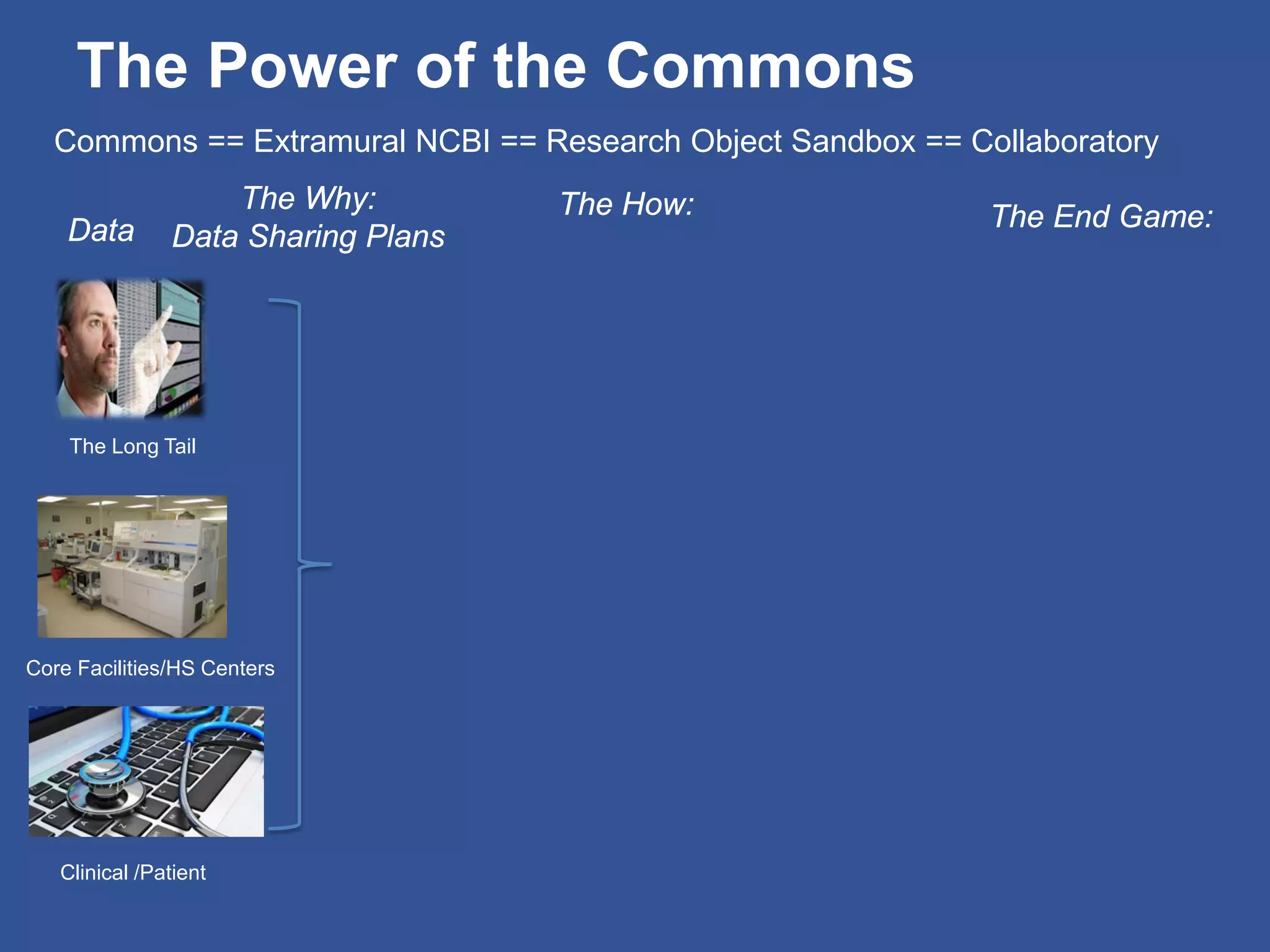
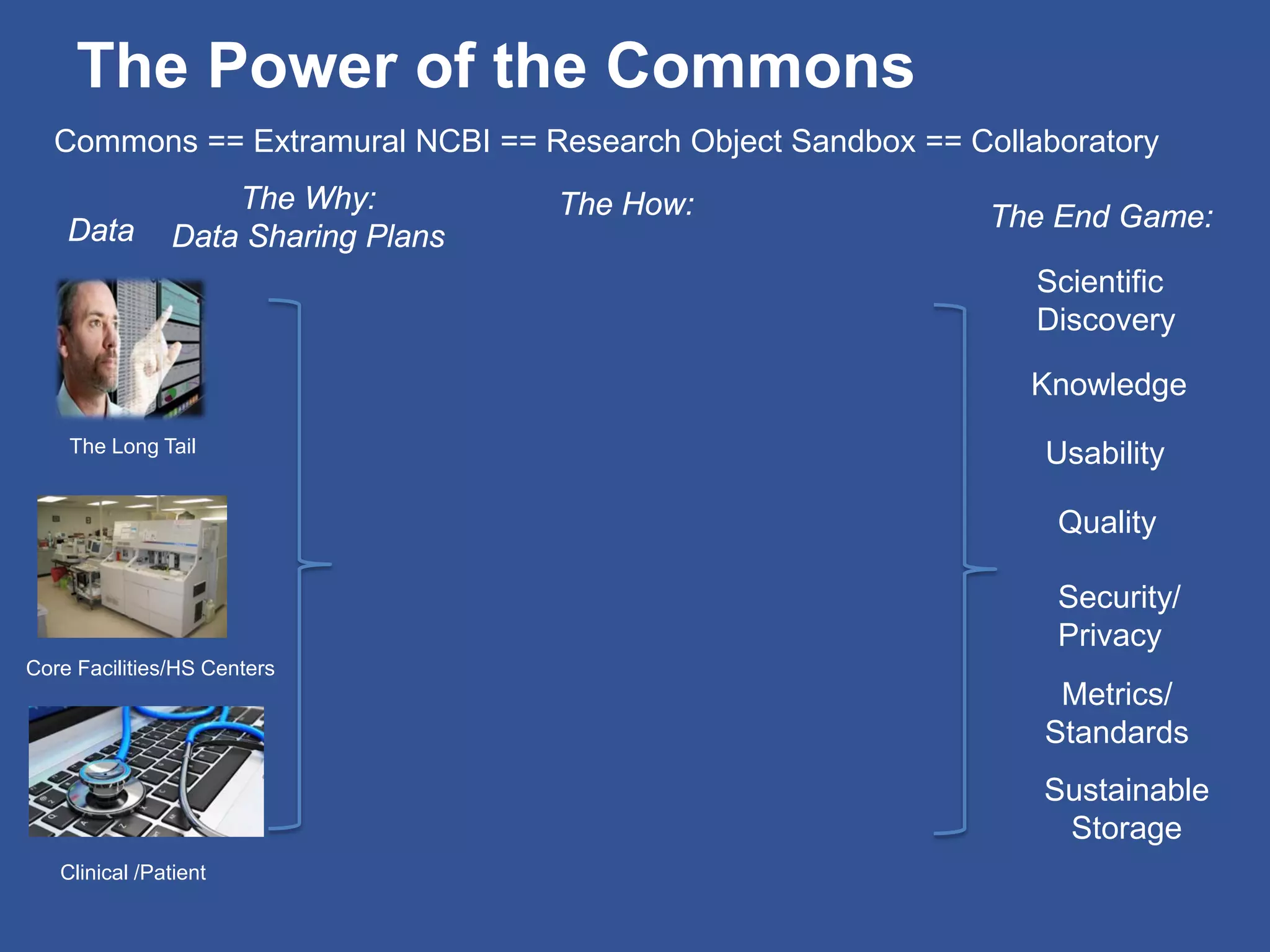
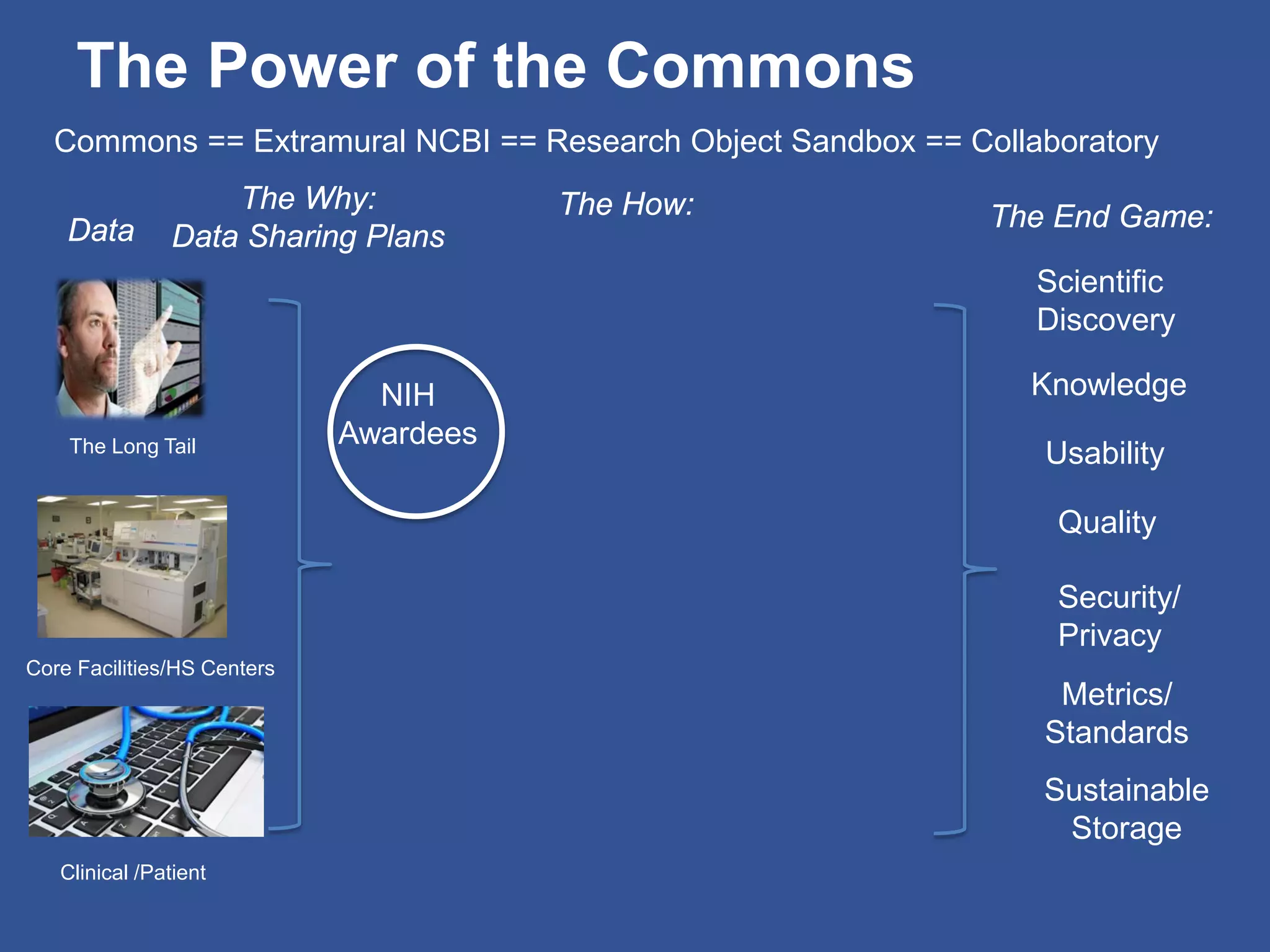
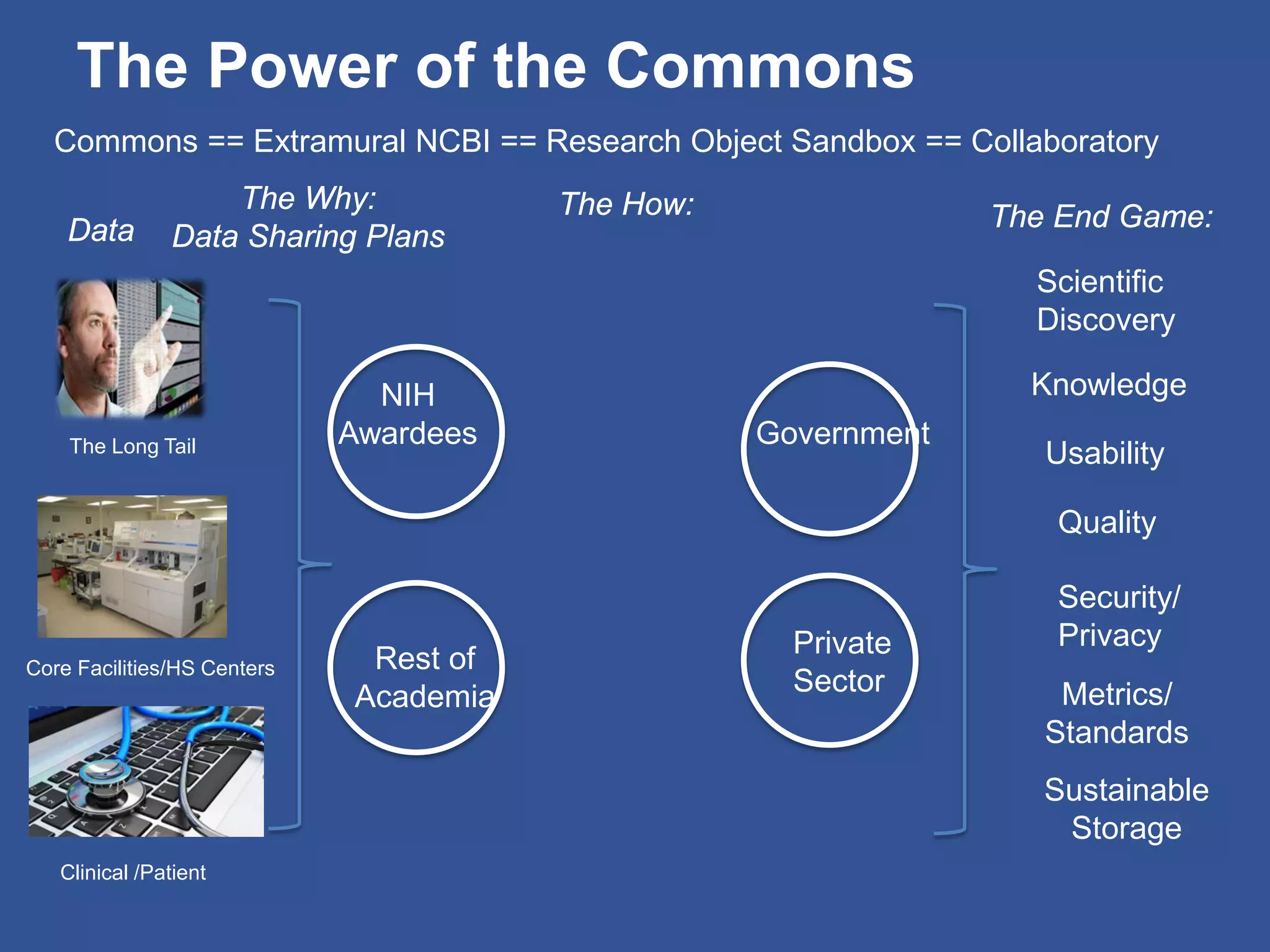
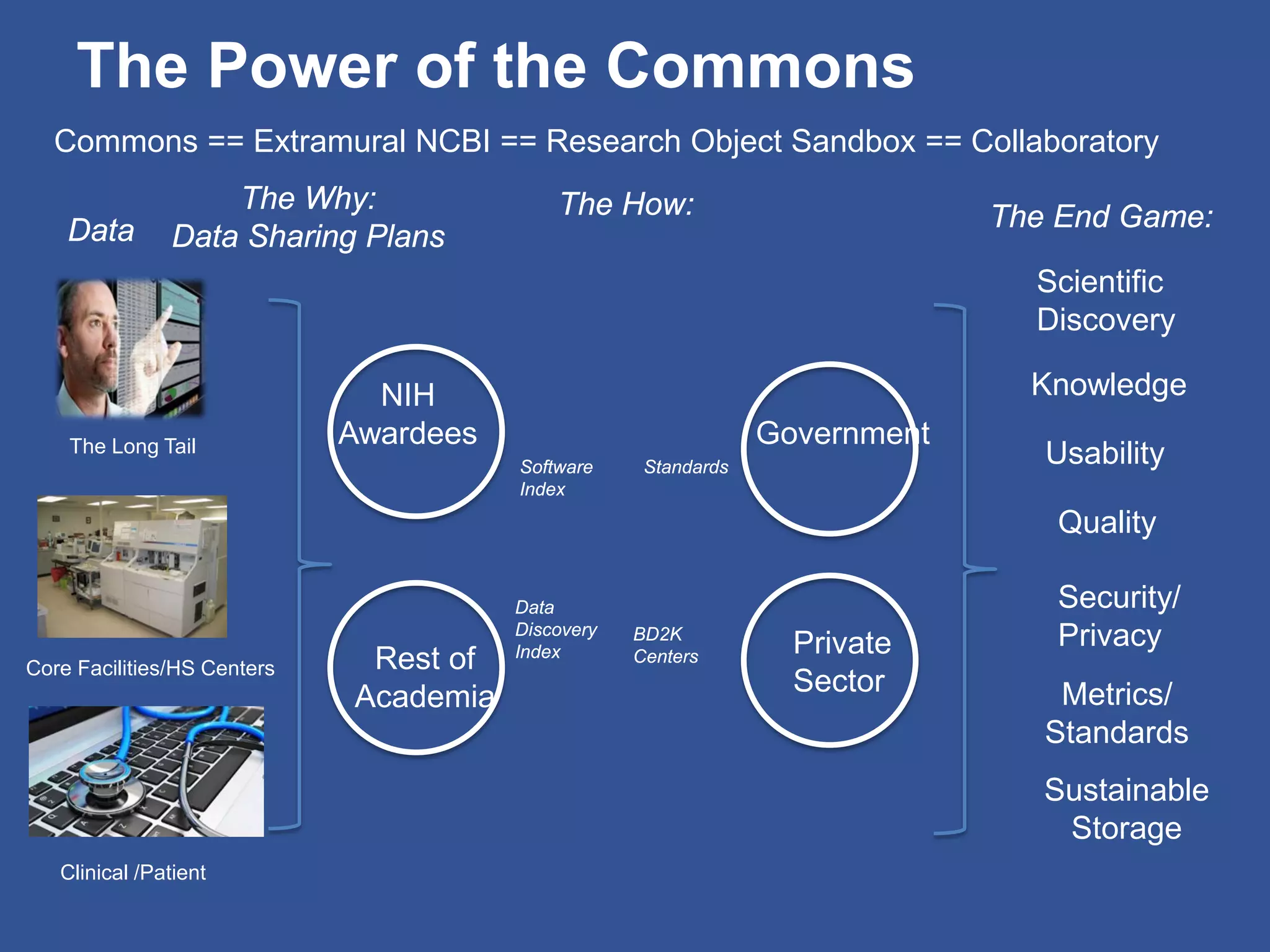
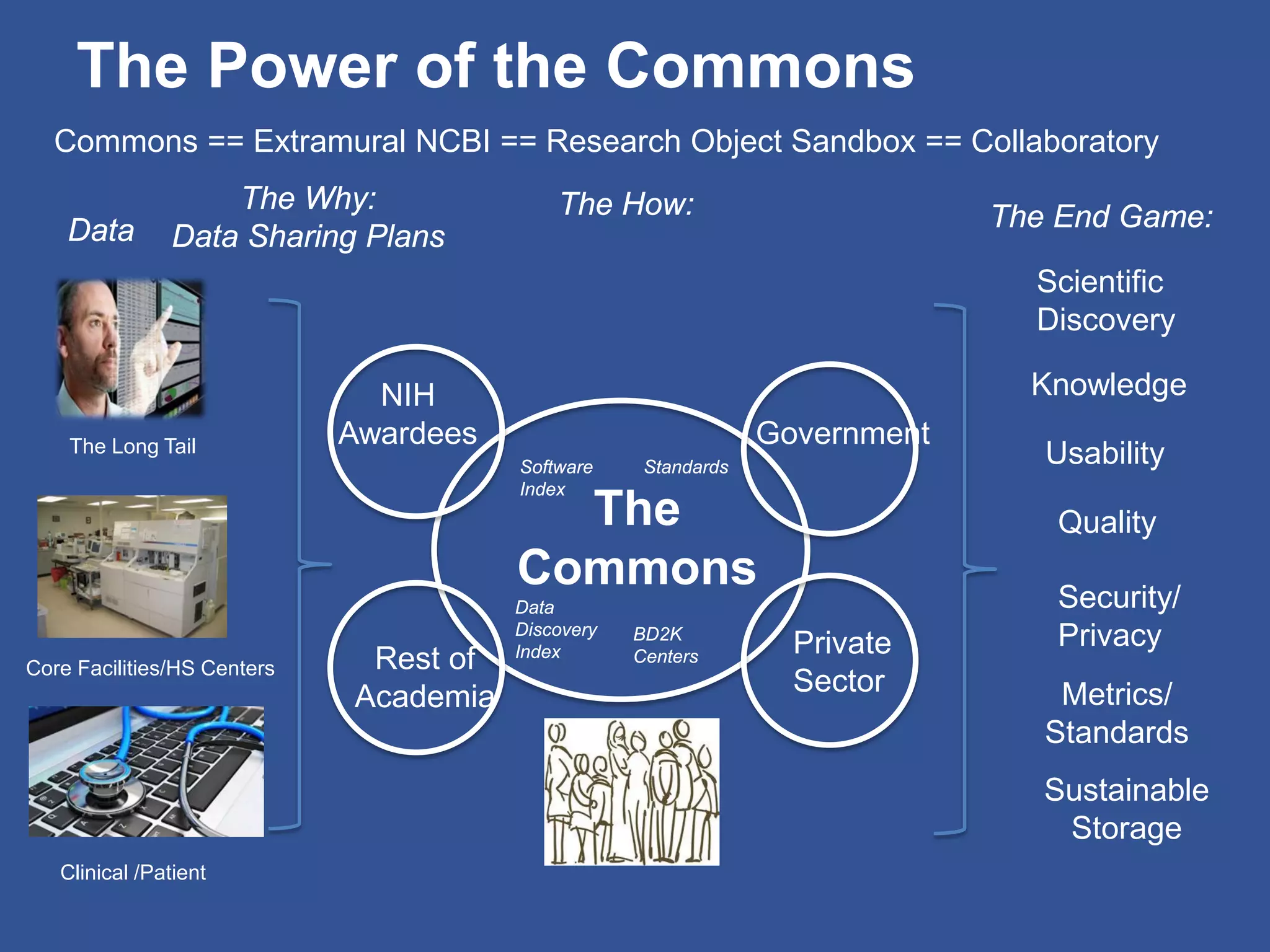
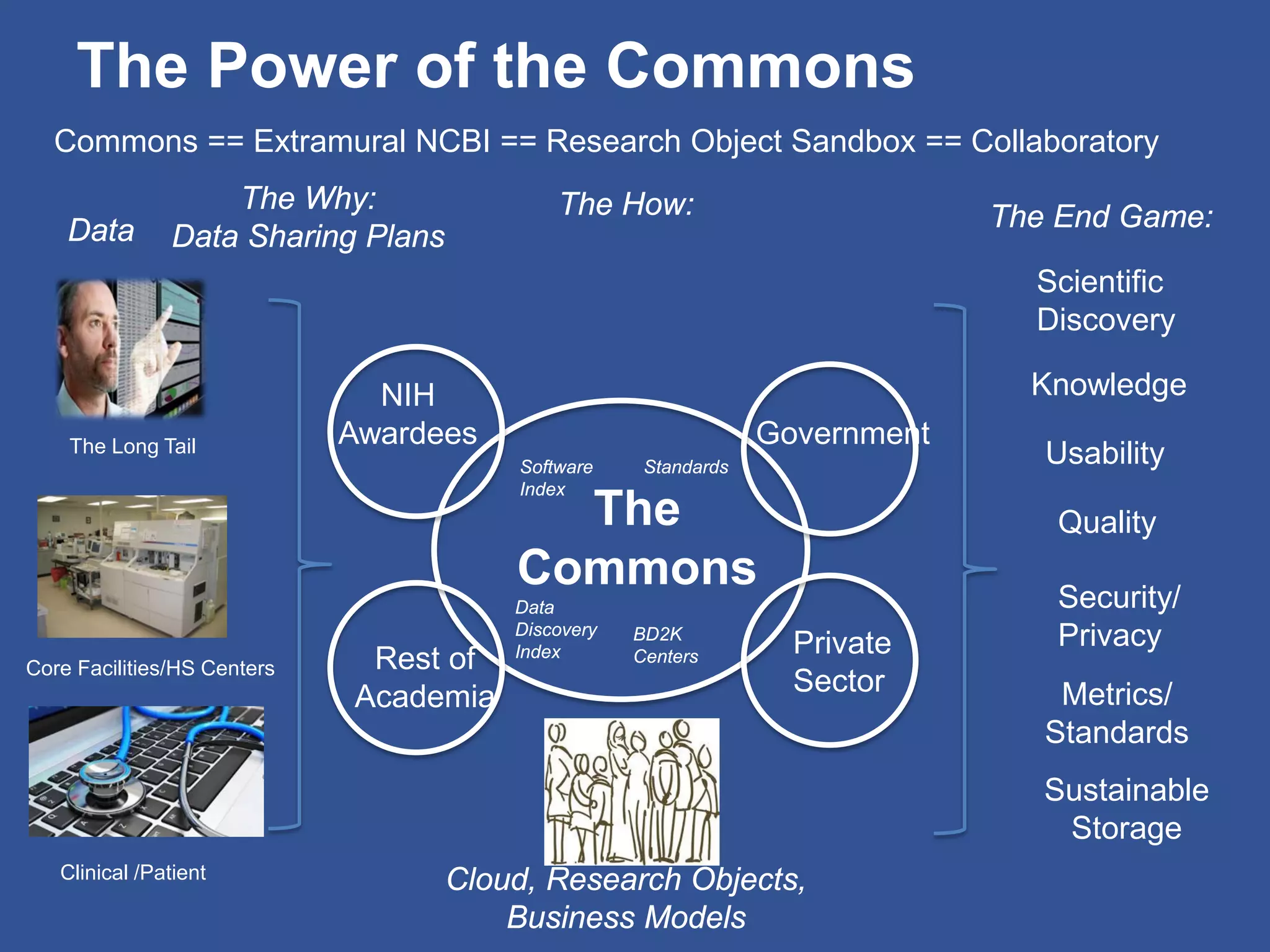
The document summarizes a panel discussion on data sharing featuring the executive directors of PCORI and NIH who discussed their organizations' efforts to build large clinical research networks and promote genomic and clinical data sharing. They addressed challenges around data standards, privacy, and incentivizing data sharing and publication of results. The associate director for data science at NIH then outlined plans to develop a biomedical research data commons to enable discovery and innovation through open data access and analytics tools.