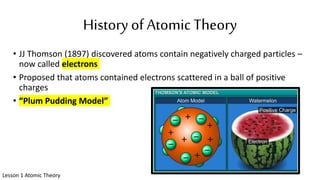
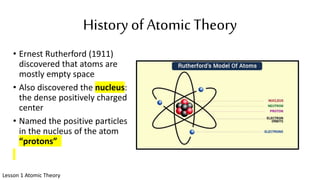
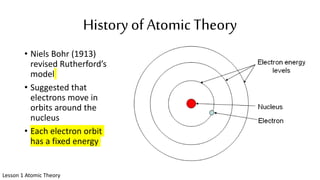
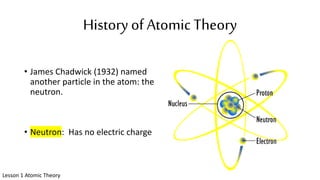
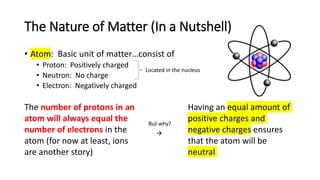
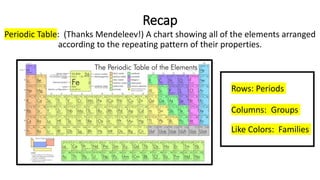
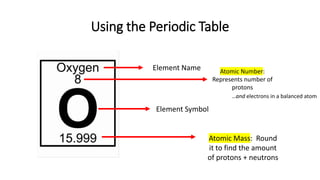
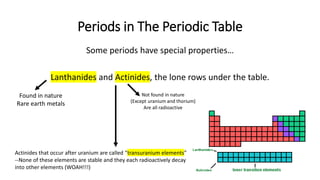
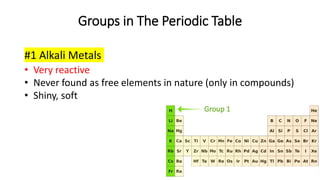
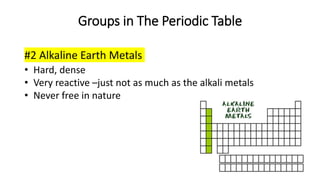
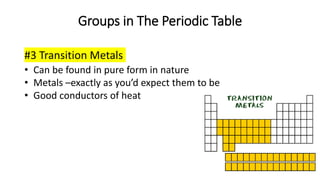
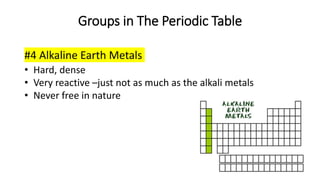
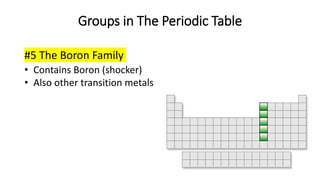
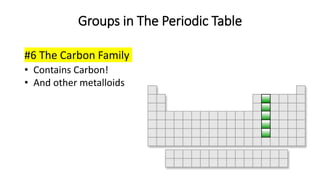
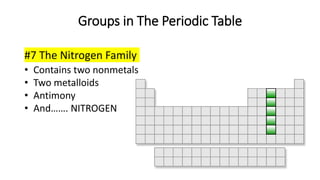
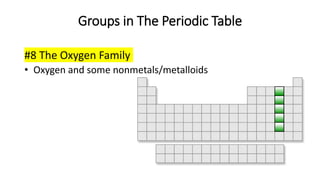
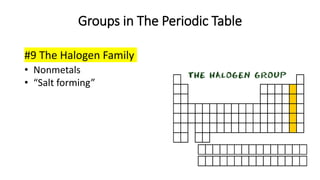
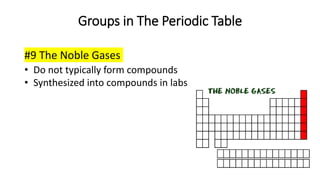
The document discusses the history of atomic theory from ancient Greek philosophers to modern scientists. It explains that Democritus first proposed the idea of atoms as indivisible pieces of matter. John Dalton further developed atomic theory in the 1700s, proposing that elements are made of unique atoms that combine in fixed ratios. J.J. Thomson, Ernest Rutherford, Niels Bohr, and James Chadwick contributed key discoveries about atomic structure through the early 1900s, such as identifying the electron and nucleus. The periodic table organizes elements based on their atomic structure and properties.