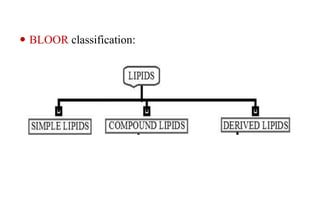
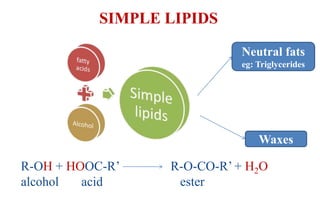
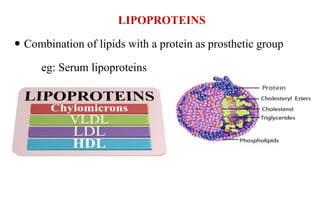
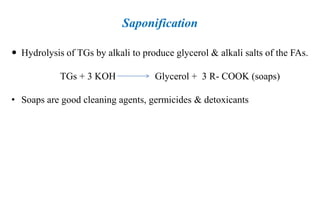
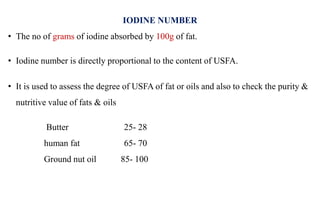
This document provides information about lipids including their definition, classification, and functions. It begins by defining lipids as naturally occurring organic and water-insoluble substances that are soluble in non-polar solvents. It then classifies lipids into simple lipids like triglycerides and waxes, and compound lipids like phospholipids, glycolipids, and lipoproteins. The document discusses the biological importance of lipids, listing functions like serving as an energy source, forming membranes, and absorbing fat-soluble vitamins. It also summarizes several tests used to characterize fats and oils, including iodine number, saponification number, and acid number.