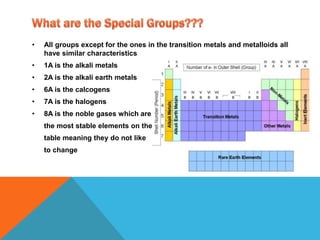
Chemistry is the study of atoms and how they combine to form compounds. Atoms are made up of protons, neutrons, and electrons. The periodic table organizes all known elements based on their atomic structure and properties. Atoms of different elements combine in multiple ways to form compounds with unique characteristics unlike the individual atoms. Understanding atomic structure and how elements interact is fundamental to chemistry and impacts many areas of science, technology, and medicine.