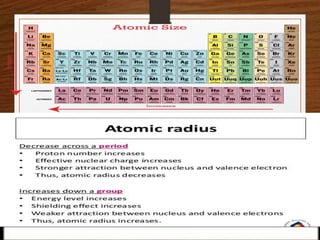
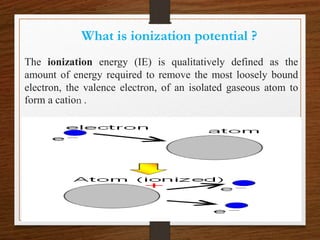
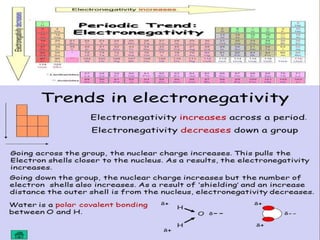
This presentation summarizes key concepts about the periodic table and periodic properties. It introduces Dmitiri Mendeleev and Henry Moseley, who developed the periodic table and periodic law. The periodic law states that when elements are arranged by increasing atomic number, elements with similar properties occur at regular intervals. Periodic properties discussed include atomic radius, ionization potential, electron affinity, and electronegativity, and how they vary across periods and groups in the periodic table.