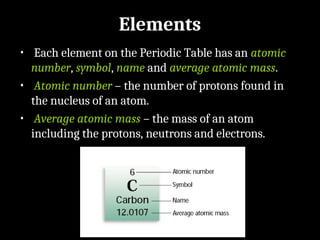
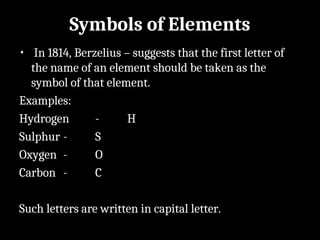
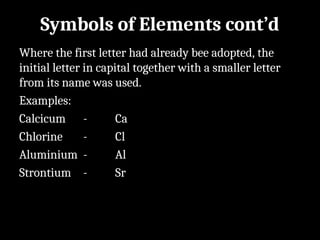
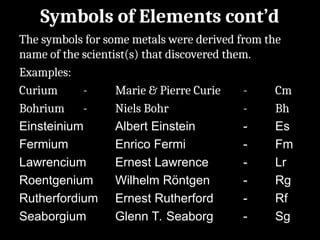
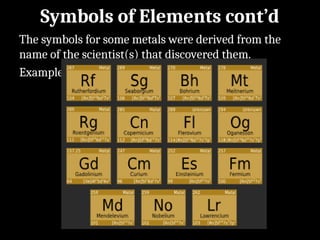
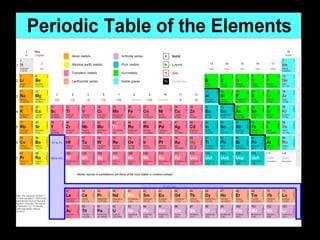
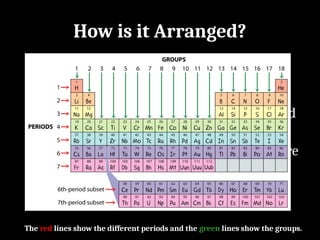
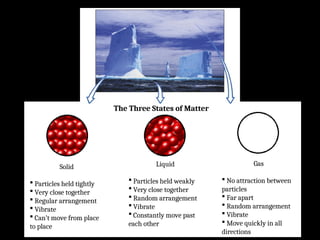
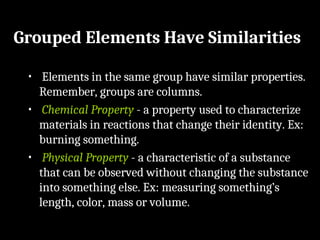
Atoms are the smallest particles made up of protons, neutrons, and electrons, with the periodic table organizing over 100 elements by atomic number and properties. Each element is defined by its atomic number, symbol, name, and average atomic mass, with symbols derived from names, scientists, and origins. The document also categorizes elements into groups based on their properties and discusses the states of matter and the characteristics of metals, non-metals, and metalloids.