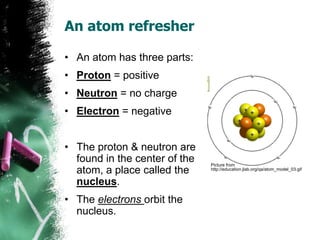
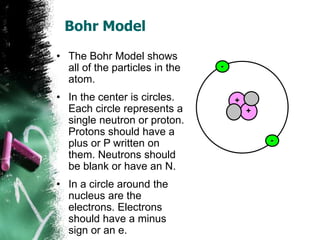
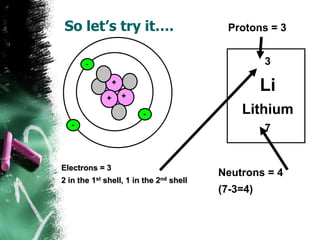
1) An atom is the basic building block of matter and is composed of protons, neutrons, and electrons. Protons and neutrons are located in the center nucleus, while electrons orbit around the nucleus.
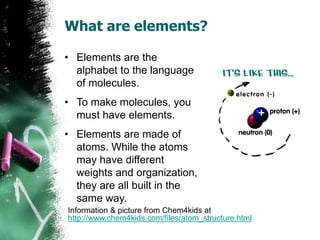
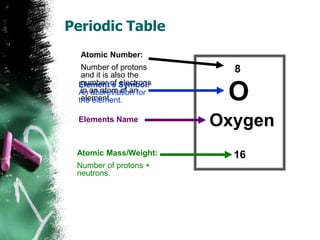
2) Elements are substances made of only one type of atom. They are the basic building blocks that make up all molecules. The periodic table lists all known elements and their properties.
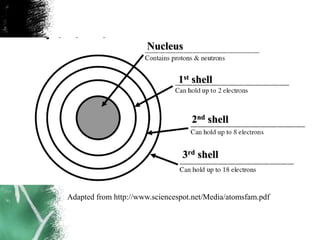
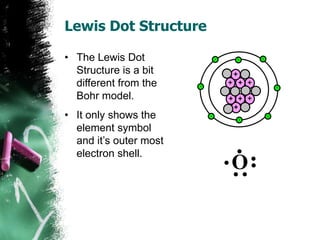
3) There are two common models of atomic structure - the Bohr model which depicts all subatomic particles, and the Lewis dot structure which represents only the outer shell electrons. Electrons are arranged into energy levels and shells around the nucleus according to specific rules.