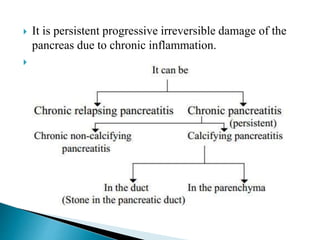
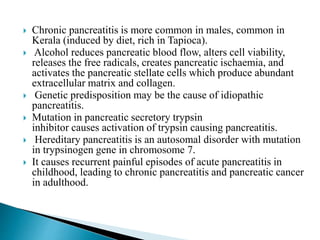
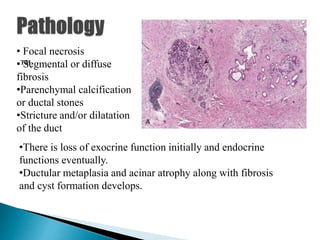
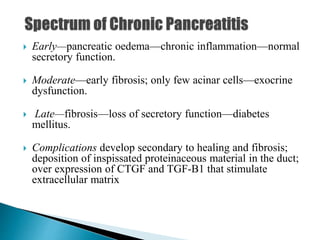
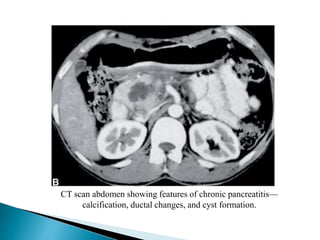
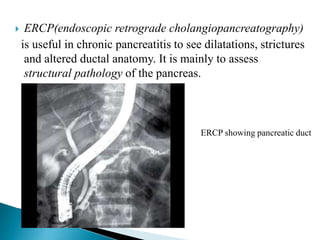
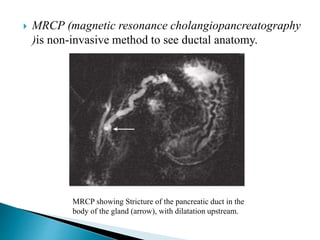
Chronic pancreatitis is persistent and progressive damage to the pancreas caused by chronic inflammation. The main causes are alcohol consumption (80% of cases) and gallstones. Genetic mutations and autoimmune disorders can also cause chronic pancreatitis. Patients present with epigastric pain, exocrine and endocrine insufficiency over time. Diagnosis involves imaging tests like CT, MRCP and EUS to identify structural changes and rule out other causes. Management focuses on pain relief and managing pancreatic insufficiency.