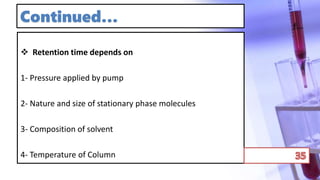
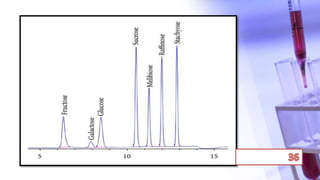
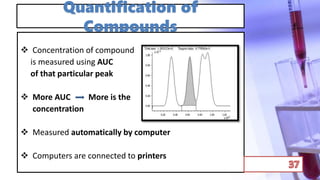
This document discusses chromatography and PCR techniques. It provides details on:
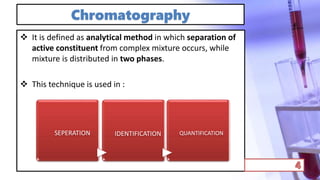
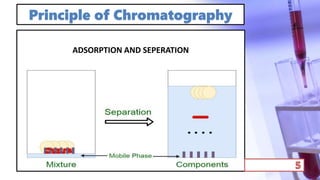
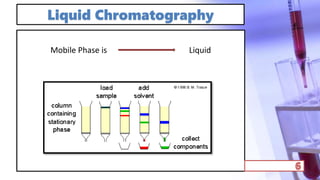
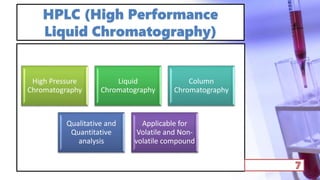
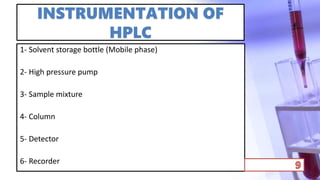
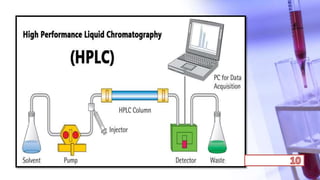
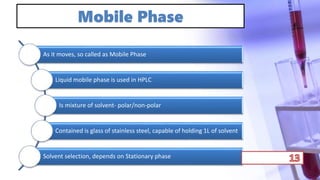
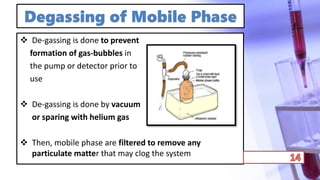
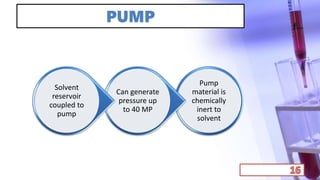
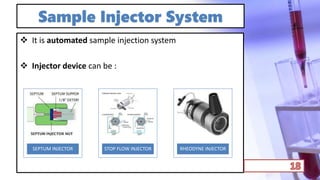
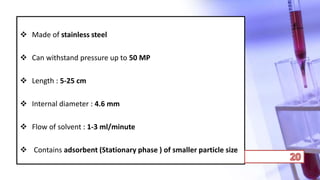
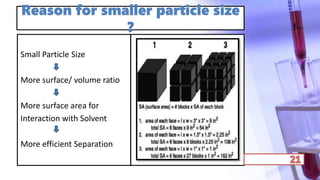
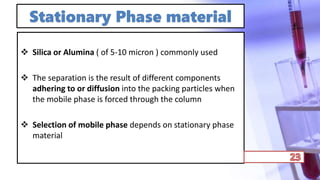
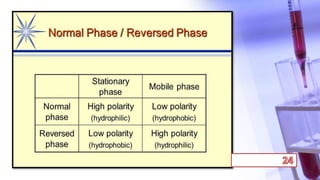
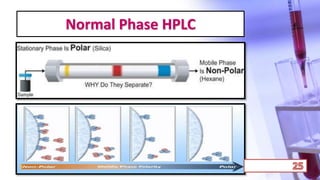
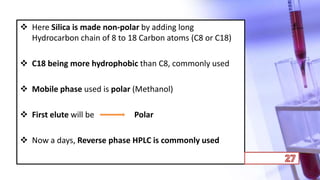
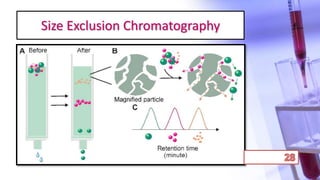
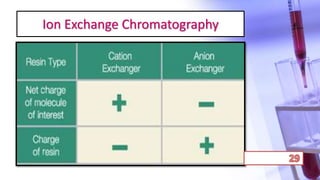
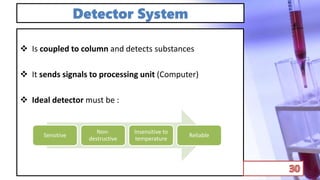
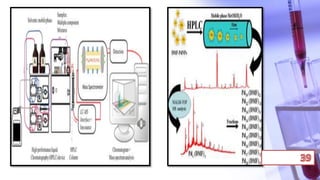
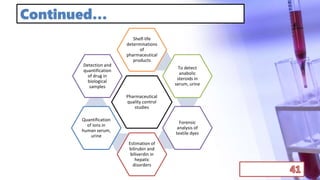
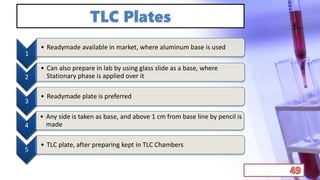
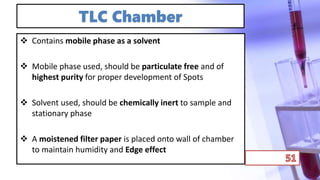
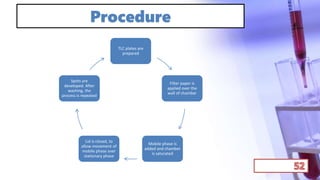
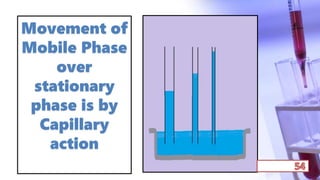
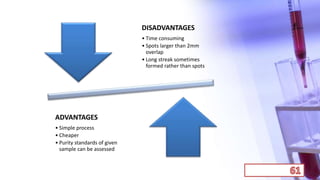
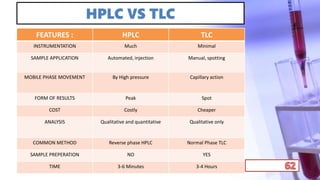
- The principles and types of chromatography including TLC, HPLC, and their components and procedures. HPLC allows for quantitative analysis and is commonly used for pharmaceutical quality control.
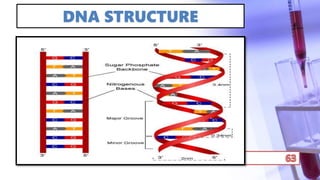
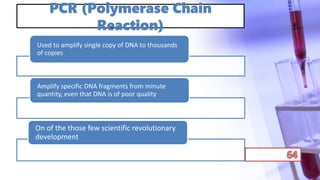
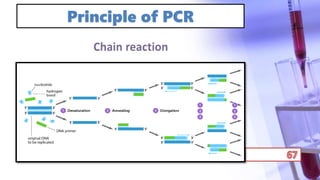
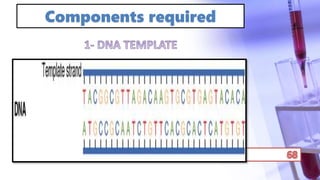
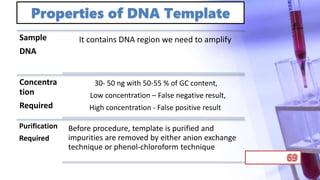
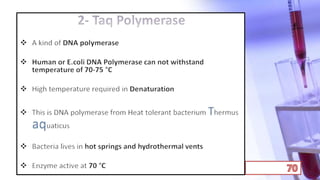
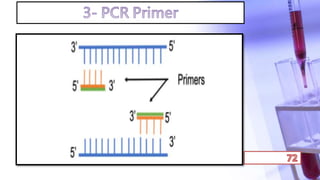
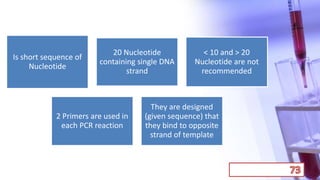
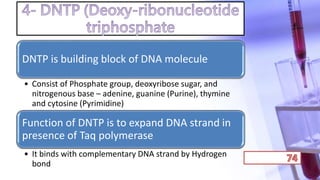
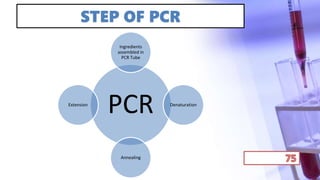
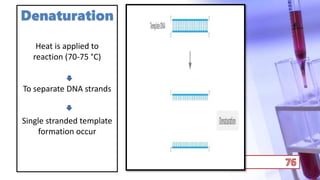
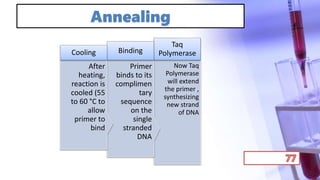
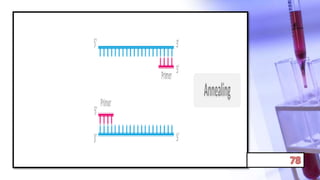
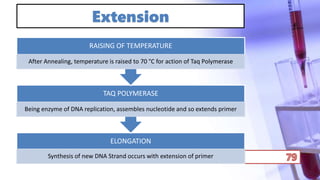
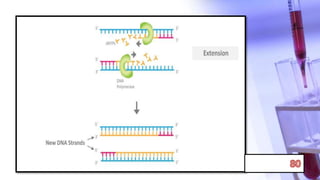
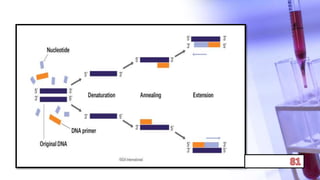
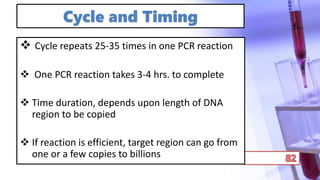
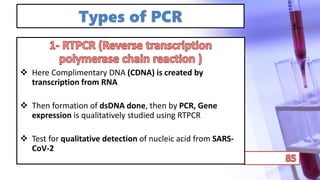
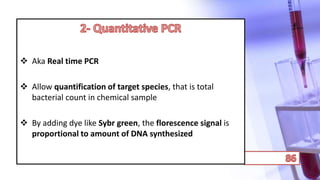
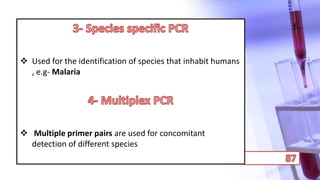
- PCR amplification which uses DNA polymerase to exponentially replicate DNA sequences. It requires template DNA, primers, nucleotides, and DNA polymerase. Repeated heating and cooling cycles allow for target DNA replication.
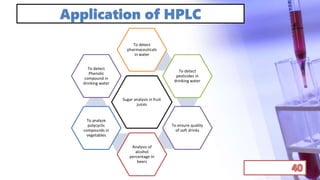
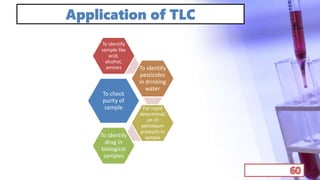
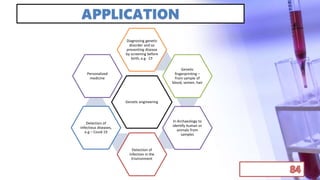
- Applications of chromatography and PCR include pharmaceutical analysis, forensic analysis, detection of genetic disorders, microbial detection, and molecular biology research techniques. Both provide powerful tools for separation, detection, and analysis of biological molecules.