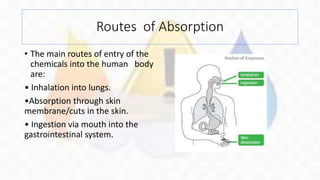
This document outlines safety standards for handling chemicals in a laboratory setting. It discusses potential hazards of chemicals, including routes of absorption into the body. Specific guidelines are provided for storing, transporting, inventorying and disposing of chemicals safely. Proper labeling and use of protective equipment is emphasized. Chemicals require special storage depending on their properties, such as flammables requiring flame-resistant cabinets. Annual inventory checks and proper disposal procedures help prevent accidents and protect health.