1. The document discusses various topics related to handling chemicals and reagents safely and properly, including classifying chemicals according to their physical and health hazards, preparing standard solutions, and labeling and storing chemicals.
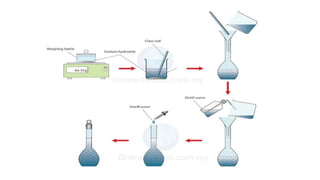
2. Primary standards are highly pure substances used to standardize secondary standards, which are prepared in the laboratory. Proper handling includes wearing protective equipment, promptly cleaning spills, and properly disposing of waste.
3. Chemicals require safe storage depending on their properties, such as storing flammables away from flames and oxidizers separately from flammables.