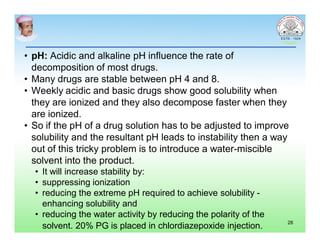
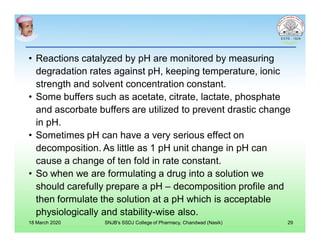
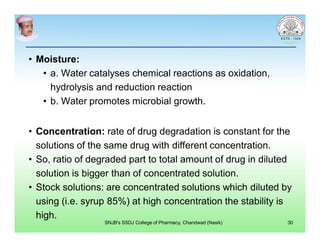
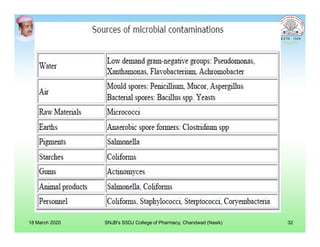
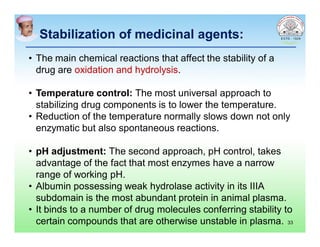
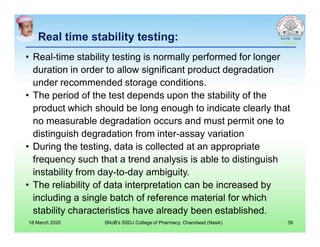
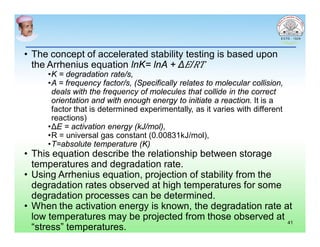
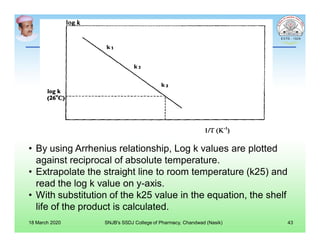
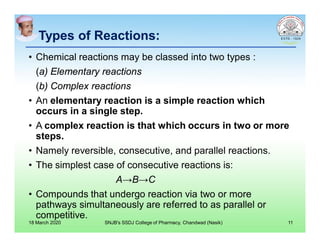
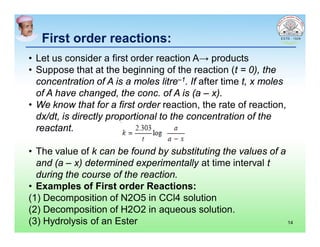
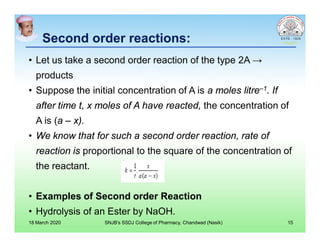
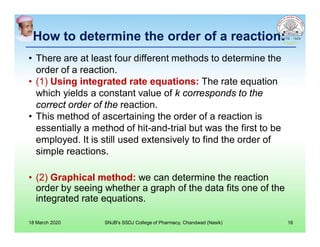
This document discusses chemical kinetics and factors that influence the degradation of pharmaceutical products. It covers topics like reaction rates, rate laws, reaction order, rate constants, and factors affecting chemical degradation. Specifically, it describes common chemical degradation pathways for drugs like hydrolysis, oxidation, isomerization, and how physical factors like temperature, solvent, and polymorphism can also cause degradation. The goal of stability testing is to determine the quality, shelf life, and recommended storage conditions for drug substances and products.

![• Rate Law: The empirical differential rate equation (or
simply the rate law) is determined experimentally and is
defined as the expression for the rate of reaction in terms of
concentrations of chemical species as indicated by
Equation
• Rate = k[reactant 1]m[reactant 2]n ....
• where k is the rate constant (or rate coefficient) and the
exponents (m) and (n) are determined experimentally and
can be a whole number (positive or negative) or, in complex
reactions, fractions.
• The reaction rate equation (RRE) contains concentration
terms for all species that interact up to and including the
rate-limiting step.
18 March 2020 SNJB's SSDJ College of Pharmacy, Chandwad (Nasik) 4](https://image.slidesharecdn.com/chemicalkinetics-dr-200318091742/85/Chemical-kinetics-dr-asm-4-320.jpg)
![Zero order reaction:Zero order reaction:
• A reactant whose concentration does not affect the reaction
rate is not included in the rate law. In effect, the
concentration of such a reactant has the power 0.
• Thus [A]0 = 1.
• A zero order reaction is one whose rate is independent
of concentration. For example, the rate law for the
reaction
• NO2 + CO ⎯⎯→ NO + CO2 at 200°C is rate = k [NO2]2
• Here the rate does not depend on [CO], so this is not
included in the rate law and the power of [CO] is
understood to be zero.
• The reaction is zeroth order with respect to CO. The
reaction is second order with respect to [NO2]. The overall
reaction order is 2 + 0 = 2.
18 March 2020 SNJB's SSDJ College of Pharmacy, Chandwad (Nasik) 12](https://image.slidesharecdn.com/chemicalkinetics-dr-200318091742/85/Chemical-kinetics-dr-asm-12-320.jpg)
![PseudoPseudo––order reactions:order reactions:
• A reaction in which one of the reactants is present in a large
excess shows an order different from the actual order. The
experimental order which is not the actual one is
referred to as the pseudo order.
• Let us consider a reaction A + B ⎯⎯→ products
• in which the reactant B is present in a large excess. Since it
is an elementary reaction, its rate law can be written as
rate = k [A] [B]
• As B is present in large excess, its concentration remains
practically constant in the course of reaction. Thus the rate
law can be written as rate = k′ [A]
• where the new rate constant k′ = k [B].
• Thus the actual order of the reaction is second-order but in
practice it will be first-order. Therefore, the reaction is said to
have a pseudo-first order. 13](https://image.slidesharecdn.com/chemicalkinetics-dr-200318091742/85/Chemical-kinetics-dr-asm-13-320.jpg)
![• (3) Using half-life period: Two separate experiments are
performed by taking different initial concentrations of a
reactant.
• The progress of the reaction in each case is recorded by
analysis. When the initial concentration is reduced to one-
half, the time is noted. Let the initial concentrations in the
two experiments be [A1] and [A2], while times for
completion of half change are t1 and t2 respectively.
• Calculation of order of reaction. We know that half-life
period for a first order reaction is independent of the initial
concentration, [A].
• We also know : half-life ∝ 1/[A] for 2nd order reaction
• half-life ∝ 1/ [A]2 for 3rd order reaction
18 March 2020 SNJB's SSDJ College of Pharmacy, Chandwad (Nasik) 17](https://image.slidesharecdn.com/chemicalkinetics-dr-200318091742/85/Chemical-kinetics-dr-asm-17-320.jpg)