Chapter 10.3: Acid and Base Solutions
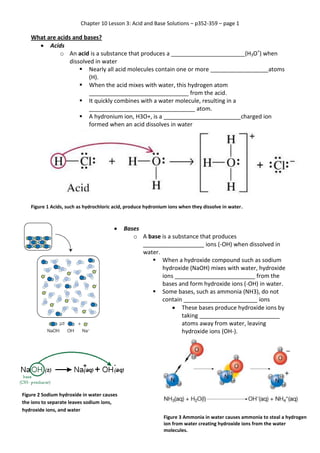
- 1. Chapter 10 Lesson 3: Acid and Base Solutions – p352-359 – page 1 What are acids and bases? Acids o An acid is a substance that produces a _______________________(H3O+) when dissolved in water Nearly all acid molecules contain one or more __________________atoms (H). When the acid mixes with water, this hydrogen atom _______________________________ from the acid. It quickly combines with a water molecule, resulting in a _________________________________ atom. A hydronium ion, H3O+, is a ________________________charged ion formed when an acid dissolves in water Figure 1 Acids, such as hydrochloric acid, produce hydronium ions when they dissolve in water. Bases o A base is a substance that produces ___________________ ions (-OH) when dissolved in water. When a hydroxide compound such as sodium hydroxide (NaOH) mixes with water, hydroxide ions _________________________ from the bases and form hydroxide ions (-OH) in water. Some bases, such as ammonia (NH3), do not contain _______________________ ions These bases produce hydroxide ions by taking _________________________ atoms away from water, leaving hydroxide ions (OH-). Figure 2 Sodium hydroxide in water causes the ions to separate leaves sodium ions, hydroxide ions, and water Figure 3 Ammonia in water causes ammonia to steal a hydrogen ion from water creating hydroxide ions from the water molecules.
- 2. Chapter 10 Lesson 3: Acid and Base Solutions – p352-359 – page 2 What is pH? The pH is an _______________________measure of the concentration of hydronium ions (H3O+) in a solution. o Inverse means that as one thing increases, another thing_____________________. o In this case, as the concentration of ______________________ions increases, pH decreases. A solution with a lower pH is more_____________________________. As the concentration of hydronium ions decreases, the pH____________________________. A solution with a higher pH is more _______________________________. Balance of Hydronium and Hydroxide Ions All acid and base solutions contain both hydronium and hydroxide ions. In a neutral solution, such as water, the concentration of hydronium and hydroxide ions are _____________________________. What distinguishes an acid from a base is which of the two ions is present in the ____________________________ concentration o ______________________have a greater concentration of hydronium ions (H3O+) than hydroxide ions (OH-) o Bases have a greater concentration of hydroxide ions than _____________________ions. Figure 4 Hydronium and Hydroxide Ion Concentration Acids o [H3O+] ______ [OH-] Neutral o [H3O+] ______ [OH-] Bases o [H3O+] ______ [OH-] *brackets around a chemical formula mean concentration The pH Scale The pH _____________________________ is used to indicate how acidic or basic a solution is. The pH scale contains values that range below _________ and above ______________ o ________________ have a pH below 7 o Bases have a pH ________________ 7 o Solutions that are neutral have a pH of _____________
- 3. Chapter 10 Lesson 3: Acid and Base Solutions – p352-359 – page 3 How is the concentration of hydronium ions different in a solution with a pH of 1 from the concentration of a solution with a pH of 2? A change in one pH unit represents a ________________________change in the acidity or basicity of a solution. o For example, if one solution has a pH of 1 and a second solution has a pH of 2, then the first solution is not twice as acidic as the second solution; it is _________________________ more acidic The _________________________ in acidity or basicity between two solutions is represented by 10n, when n is the difference between the two pH values. o For example, how much more acidic is a solution with a pH of 1 than a solution with a pH of 3? First, calculate the difference, n, between the two pH values: n = 3-1 = _____ Then use the formula 10^n, to calculate the difference in acidity: 102= _____________ A solution with a pH of 1 is _________ times more acidic than a solution with a pH of 3
- 4. Chapter 10 Lesson 3: Acid and Base Solutions – p352-359 – page 4 How is pH Measured? pH Indicators An indicator is a compound that changes _______________ at different pH values when it reacts with acidic or basic solutions o When one or two drops of indicator are added to a solution, the color of the solution changes. This color can be matched to a set of standard colors that correspond to pH. For example, bromthymol blue is an indicator that changes from ______________to green to blue between pH 6 and pH 7.6 pH Testing Strips pH can also be measured using pH testing strips The strips contain an indicator that changes to a variety of ________________ over a range of pH values These are most commonly used to test water pH Meters Although pH strips are quick and easy, they provide only an _____________________ pH value. A more accurate way to measure pH is to use a pH meter A pH meter is an electronic instrument with an ____________________that is sensitive to the hydronium ion concentration in solution.
- 5. Chapter 10 Lesson 3: Acid and Base Solutions – p352-359 – page 5 Review Questions from the Scope 1. What happens when acids and bases dissolve in water? 2. How does the concentration of hydronium ions affect pH? 3. What methods can be used to measure pH?
