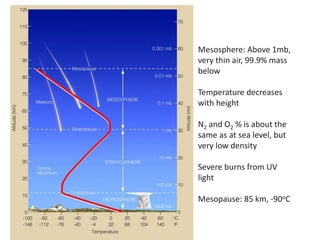
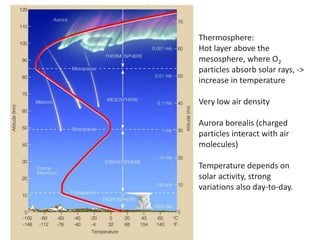
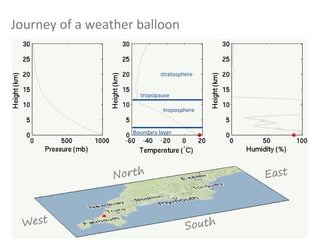
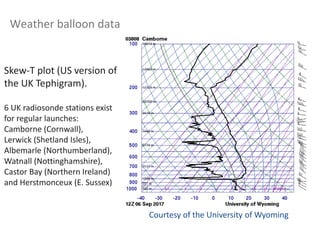
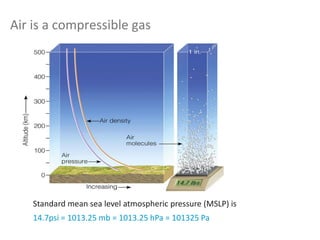
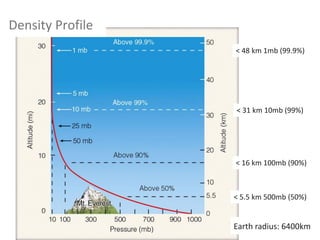
The document provides an overview of the Earth's atmosphere, detailing its composition, formation, and structure. It discusses the atmosphere's crucial role in supporting life, weather patterns, and the variations in gas concentrations over time. Additionally, it describes the vertical structure of the atmosphere, including the different layers and their properties that are vital for weather and life on Earth.
![Lecture 1
The Earth and its Atmosphere
Overview
Composition
[Radiosonde launch]
Vertical Structure](https://image.slidesharecdn.com/l1composition2017-240709092517-88066713/75/composition_function_structure_of_Atmosphere-pdf-1-2048.jpg)



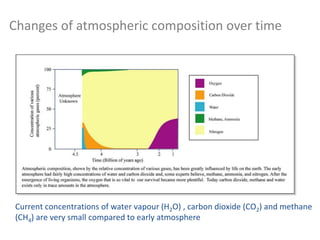



![Composition of the Atmosphere
99% of the atmosphere is within 30 km of the Earth’s surface
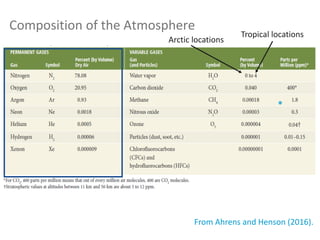
N2 78% and O2 21% (of dry air, fairly constant up to 80 km)
“Permanent”: The percentages represent a constant amount of gas
with cycles of destruction and production constantly maintaining
this amount.
N2: Denitrification mostly via biological processes (food chain)
returned to atmosphere by decaying of plant and animal matter
02: Organic matter decays, oxidation, respiration
returned to atmosphere e.g. photosynthesis
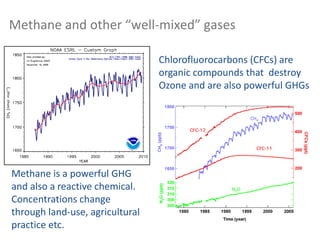
Many other gases are present with concentrations that vary on a
variety of timescales. Examples? [time out]](https://image.slidesharecdn.com/l1composition2017-240709092517-88066713/85/composition_function_structure_of_Atmosphere-pdf-9-320.jpg)
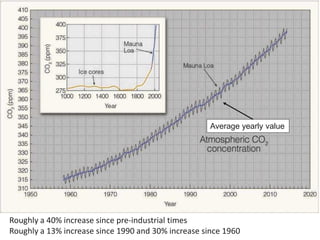
![Carbon Dioxide
Carbon Dioxide is a well-mixed gas but its concentration
changes over time.
Estimates are that the oceans hold more than 50 times the
CO2 of the atmosphere.
It has increased since pre-industrial times due to the burning
of fossil fuels.
How much has CO2 increased (in %) since:
1990 ?
1960 ?
1700 ?
[time out]](https://image.slidesharecdn.com/l1composition2017-240709092517-88066713/85/composition_function_structure_of_Atmosphere-pdf-11-320.jpg)


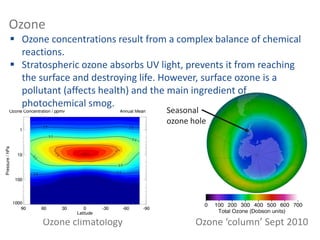









![What is happening here? [time out]
A temperature inversion (temperature
increase with height) prevents ascent of air](https://image.slidesharecdn.com/l1composition2017-240709092517-88066713/85/composition_function_structure_of_Atmosphere-pdf-32-320.jpg)