Embed presentation
Downloaded 47 times


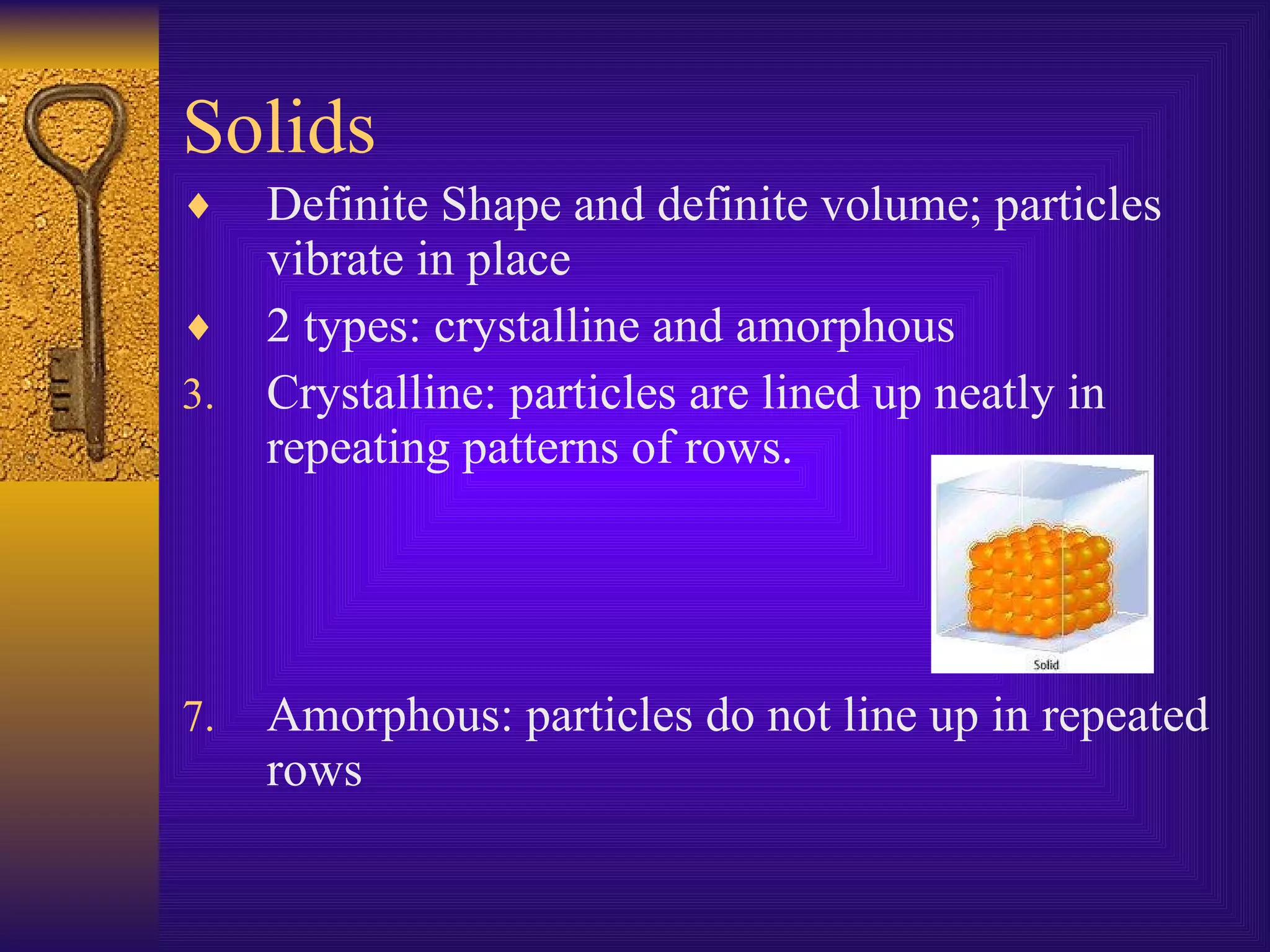




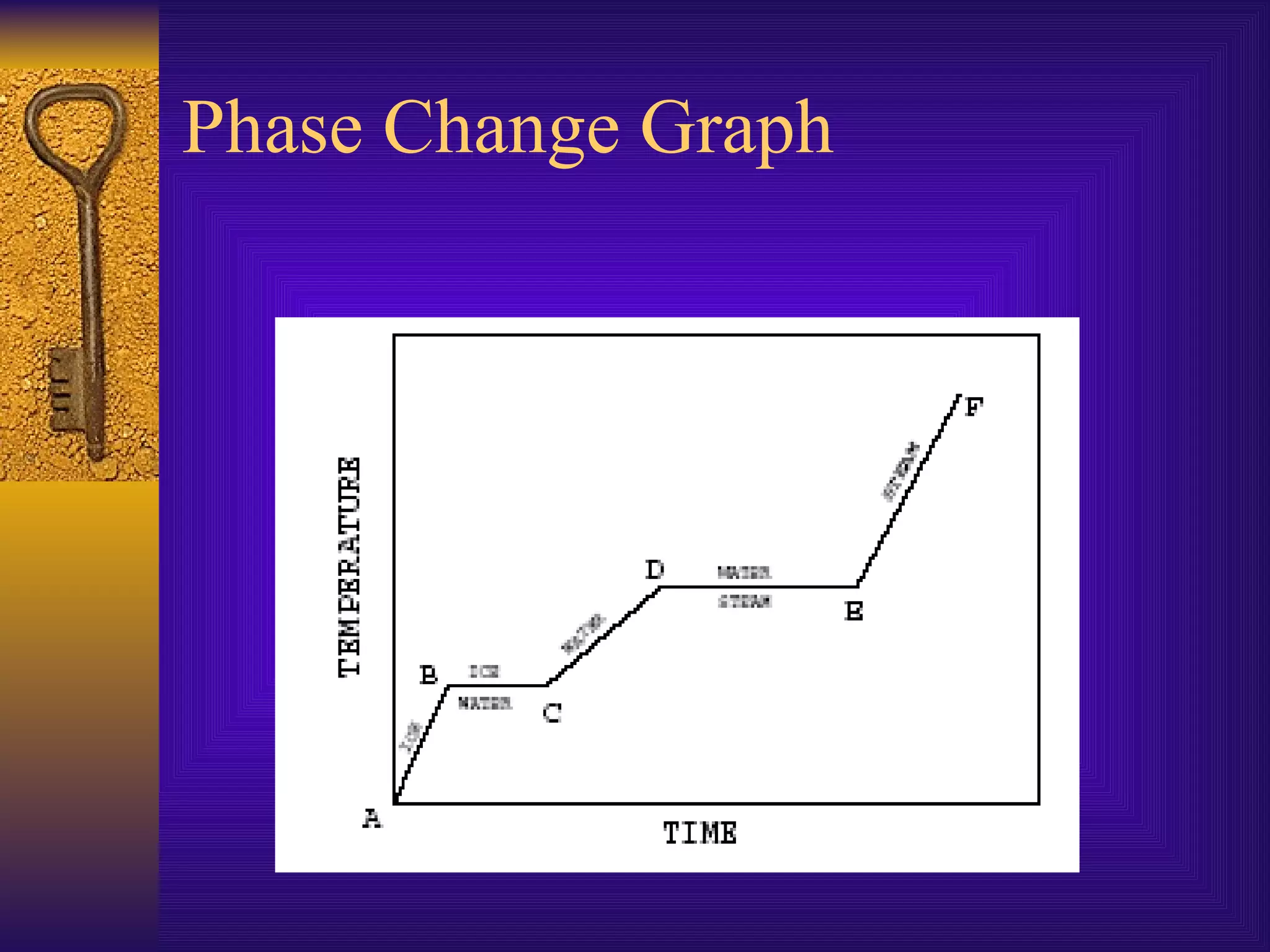
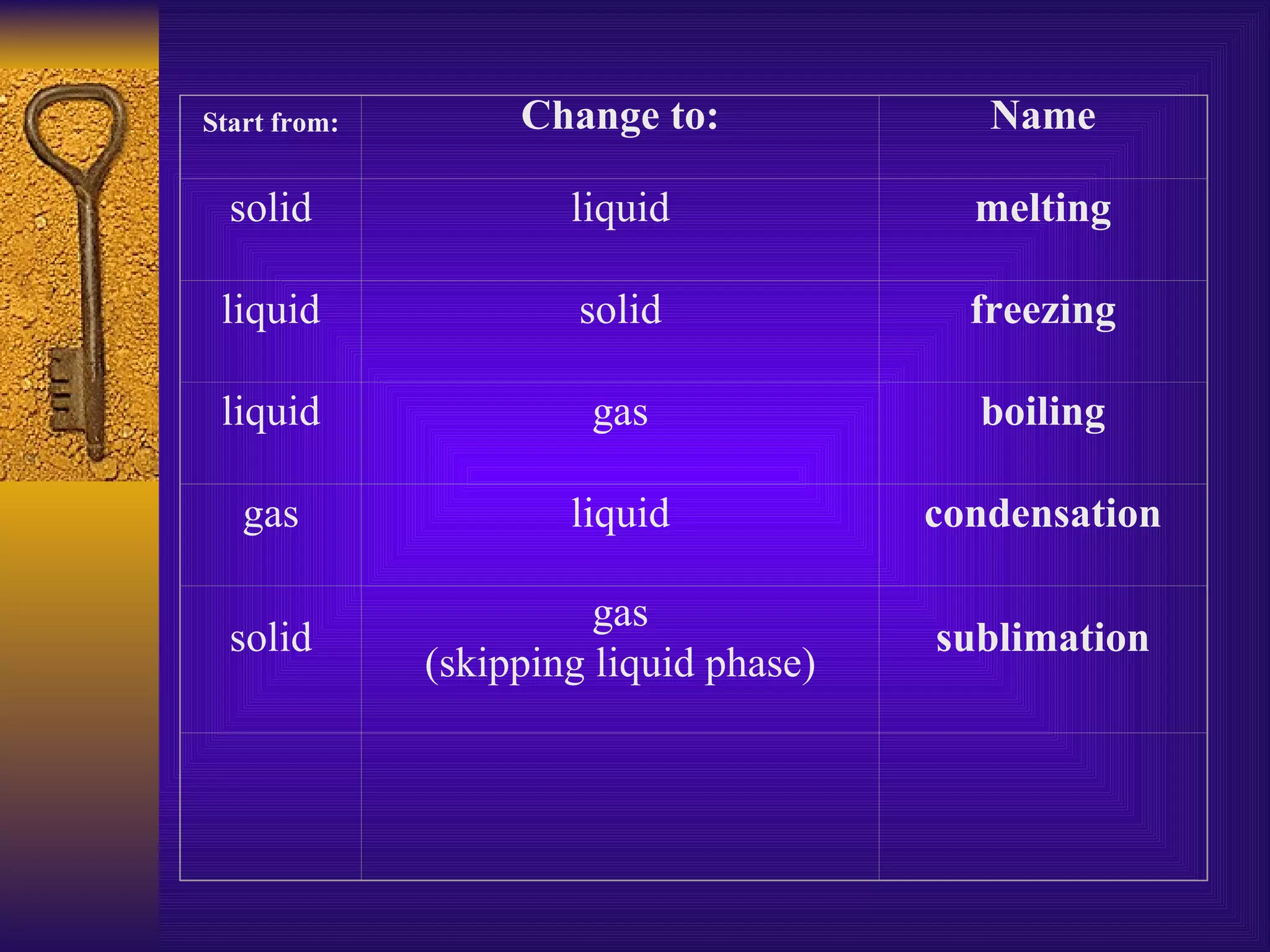
There are four main states of matter: solids, liquids, gases, and plasma. Solids have a definite shape and volume, with particles vibrating in a fixed position. Liquids have a definite volume but indefinite shape, as particles slide past one another. Gases have indefinite shapes and volumes, as particles move rapidly away from each other. Plasma is the hottest state and consists of ionized gas particles that are electrically charged.









