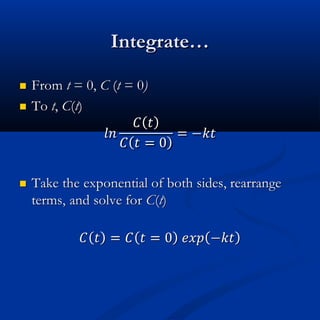
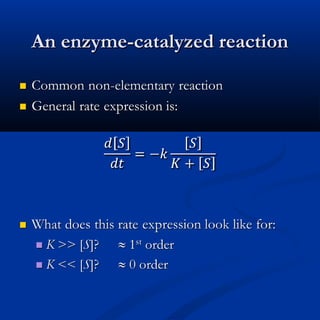
The document discusses corrections made to tables in a textbook on chemical kinetics. Table 1.1 corrected the concentration of Mg2+ and noted analytical errors in data for the Mississippi River sample that will be replaced. Table 1.3a corrected the coefficient in the Davies equation from 0.2 to 0.3, as suggested by Davies. The rest of the document covers an overview of chemical kinetics including expressions for irreversible reactions, the effects of temperature, and reversible reactions.













![Example
Apply differential approach
t (min) [A] (mg/L)
0 10
2 5.8
4 3.7
6 2.6
8 1.9
10 1.5](https://image.slidesharecdn.com/chapter2kinetics-120830143455-phpapp02/85/Chapter-2-kinetics-15-320.jpg)
![Example
Data manipulation
t (min) [A] (mg/L) ∆[A]/∆t [A]avg Ln(-∆[A]/∆t)
0 10 - -
2 5.8 -2.1 7.9 0.74
4 3.7 -1.05 4.75 0.049
6 2.6 -0.55 3.15 -0.598
8 1.9 -0.35 2.25 -1.05
10 1.5 -0.02 1.7 -1.61
[A]avg = average concentration during the period ∆t.](https://image.slidesharecdn.com/chapter2kinetics-120830143455-phpapp02/85/Chapter-2-kinetics-16-320.jpg)





























![How does water temperature
affect pH?
Equilibrium expression:
{H+} {OH-} = Kw = 10-14.0 (T = 25 C)
Charge balance in pure water:
[H+] = [OH-]
What is the pH at T = 1 °C?](https://image.slidesharecdn.com/chapter2kinetics-120830143455-phpapp02/85/Chapter-2-kinetics-47-320.jpg)


