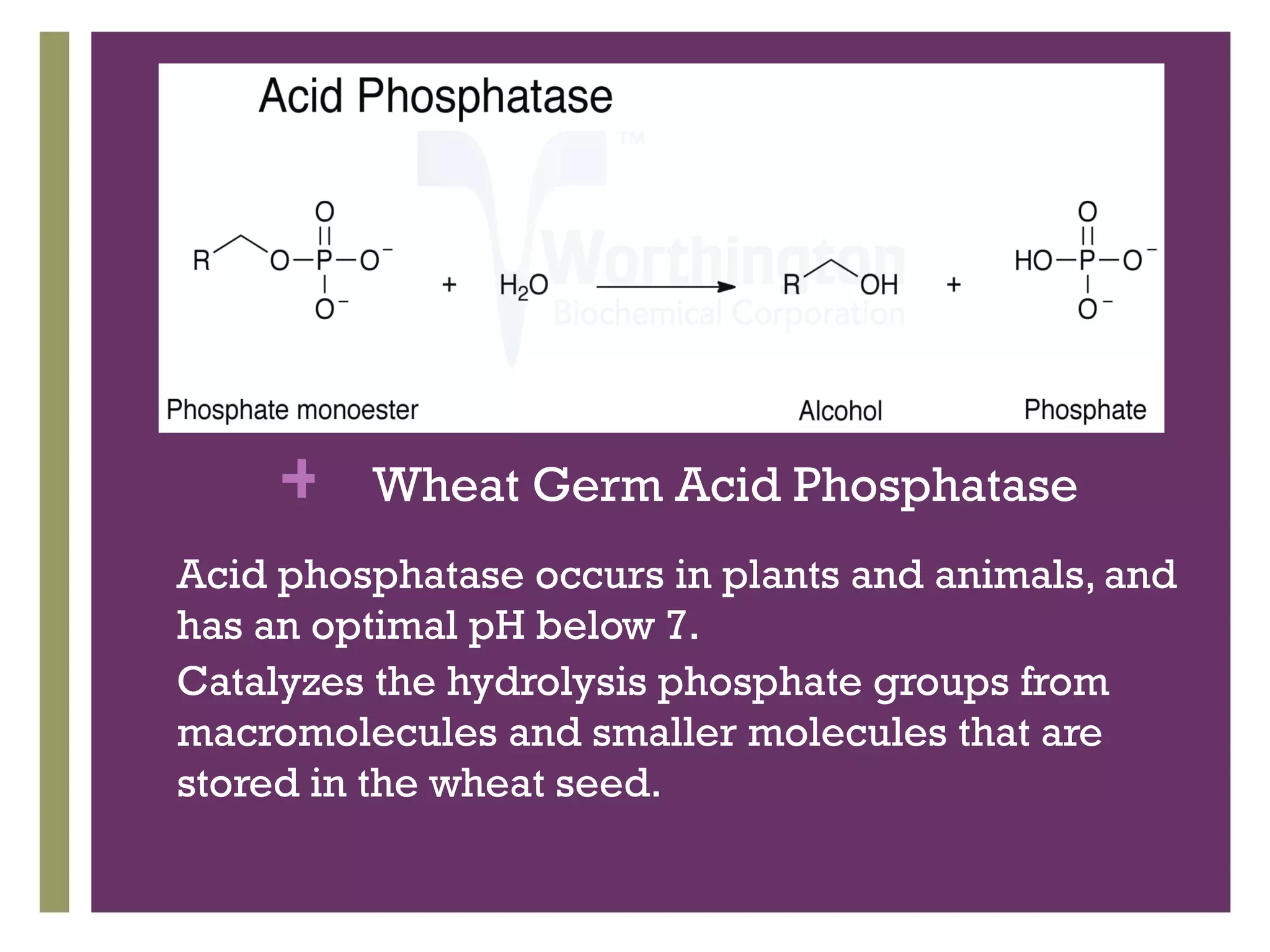
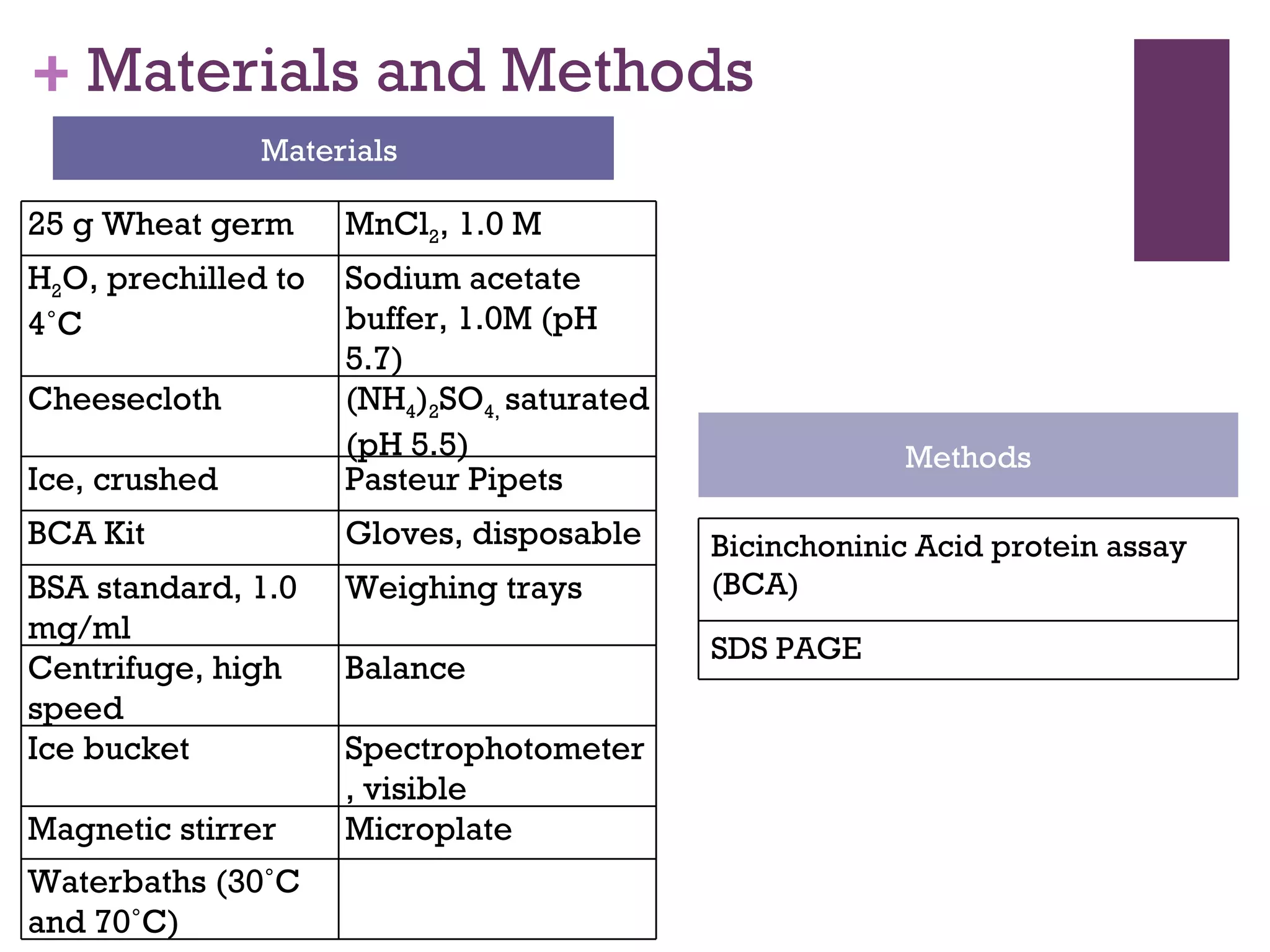
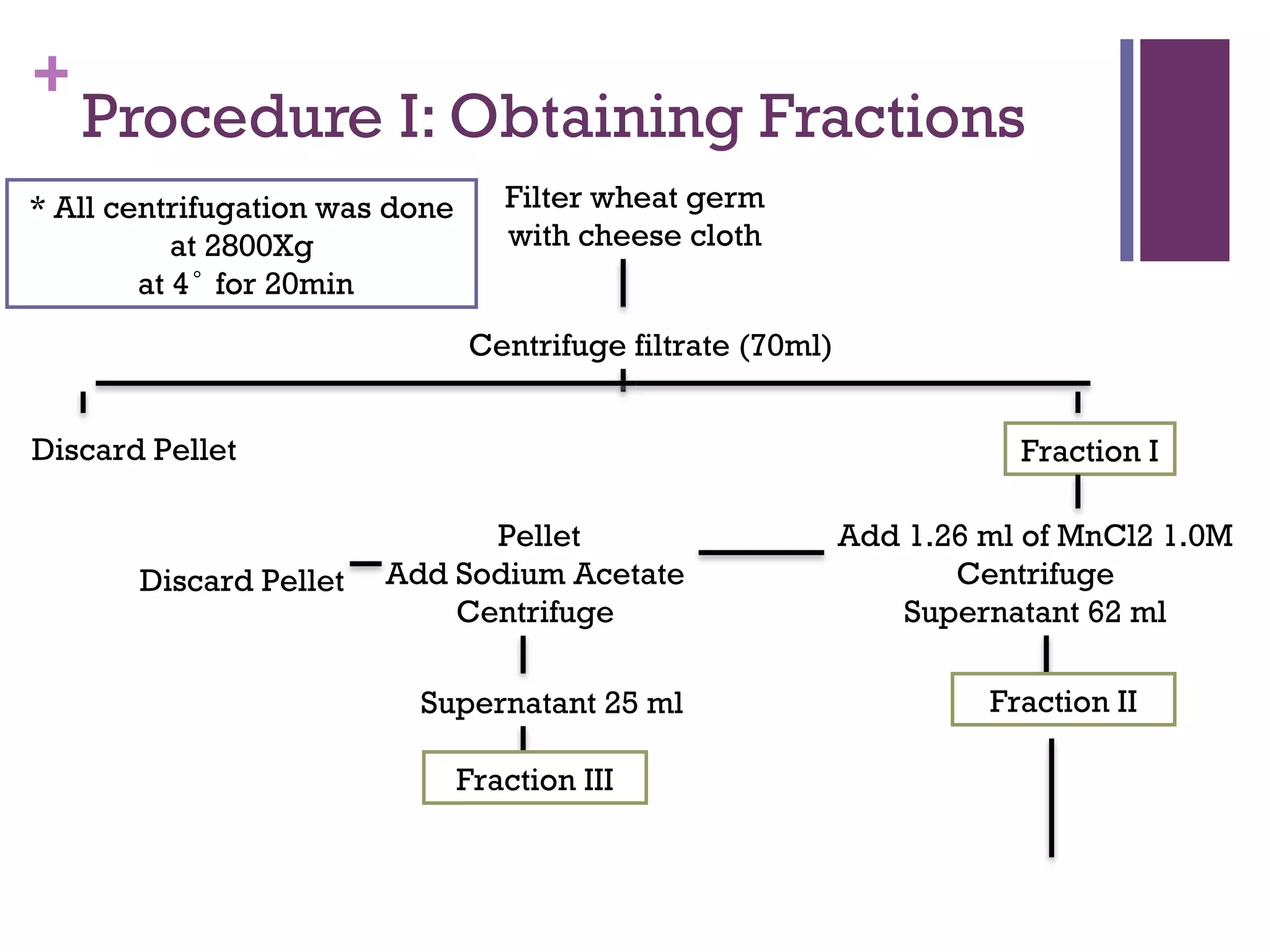
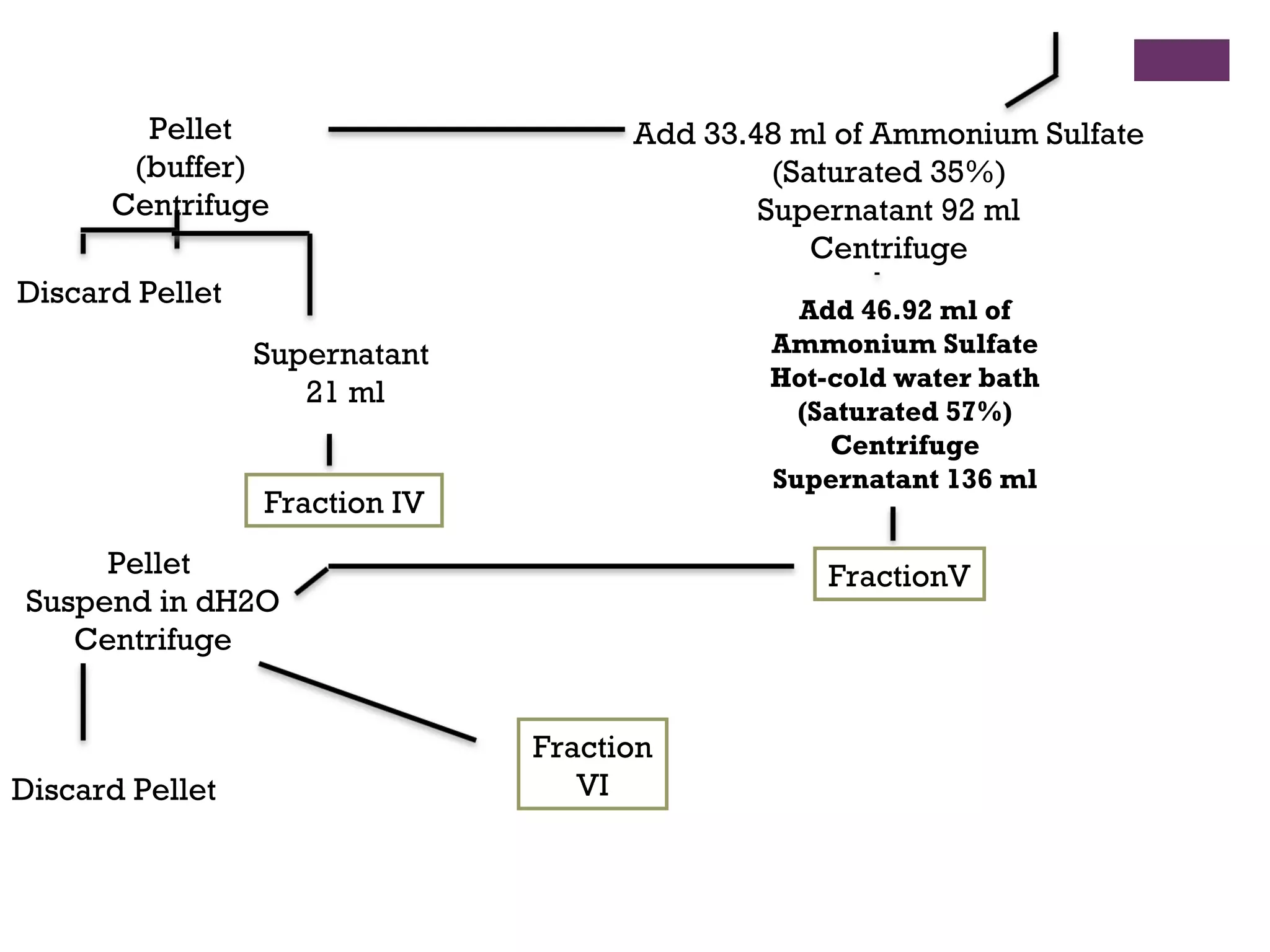
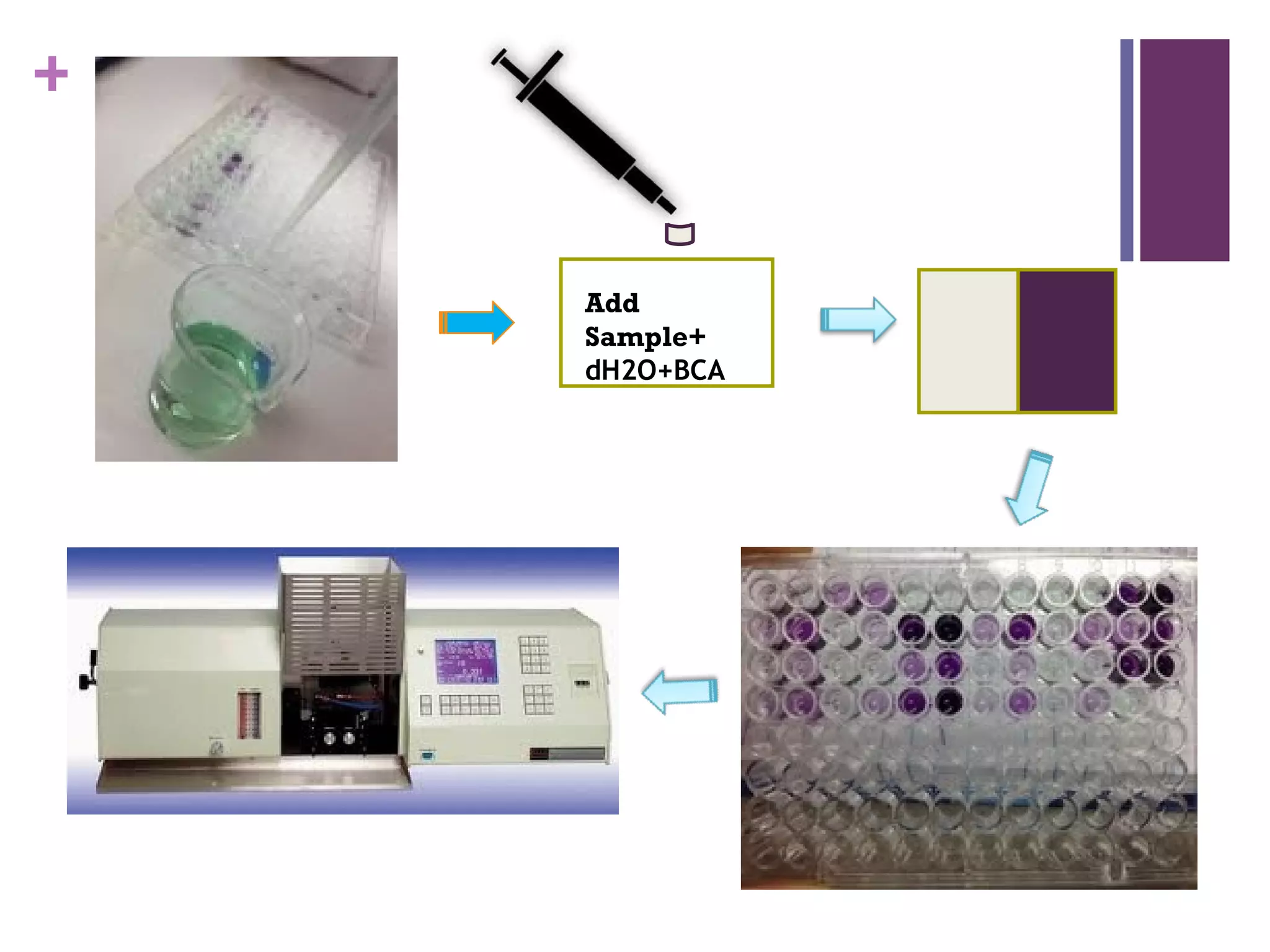
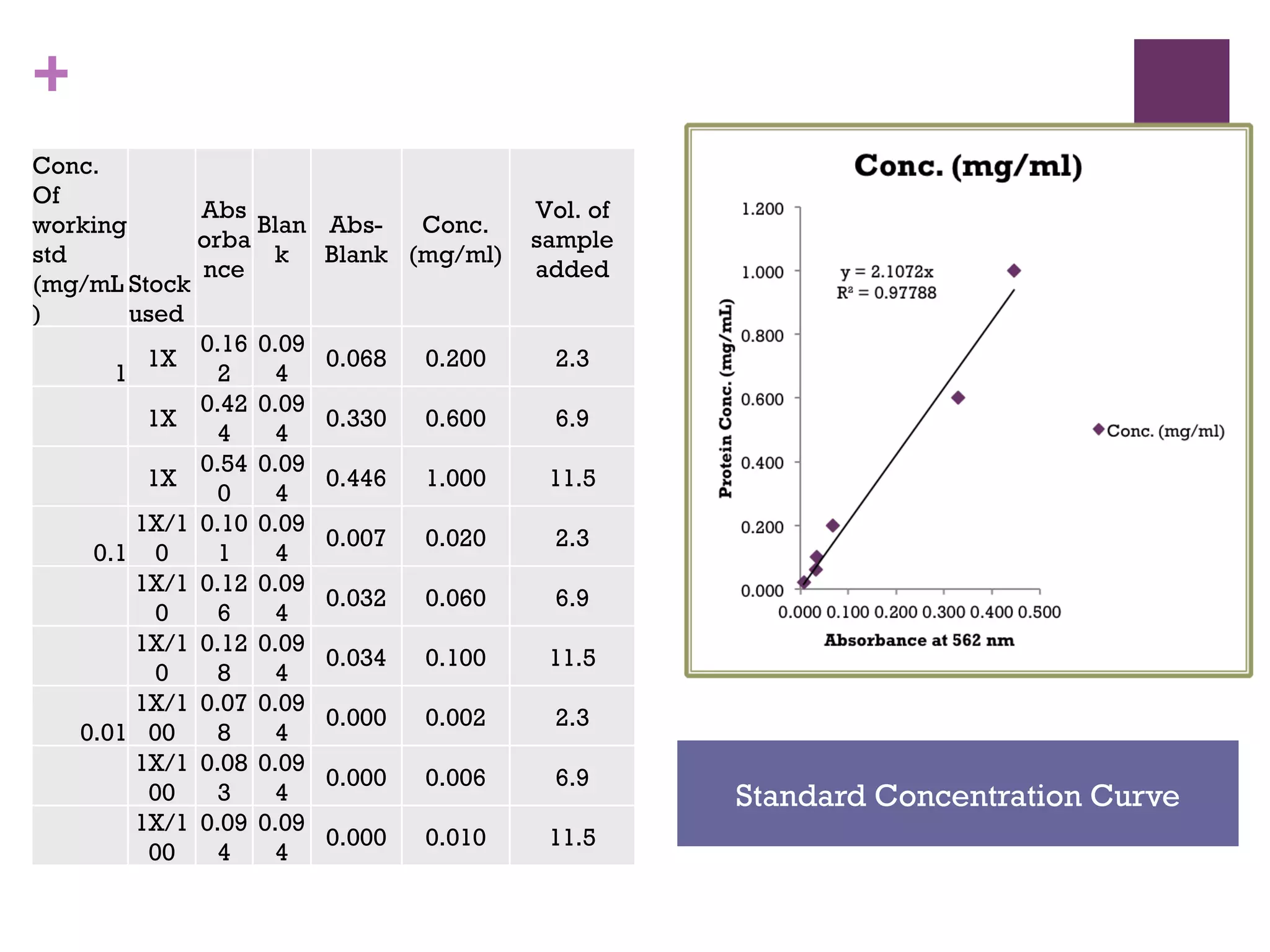
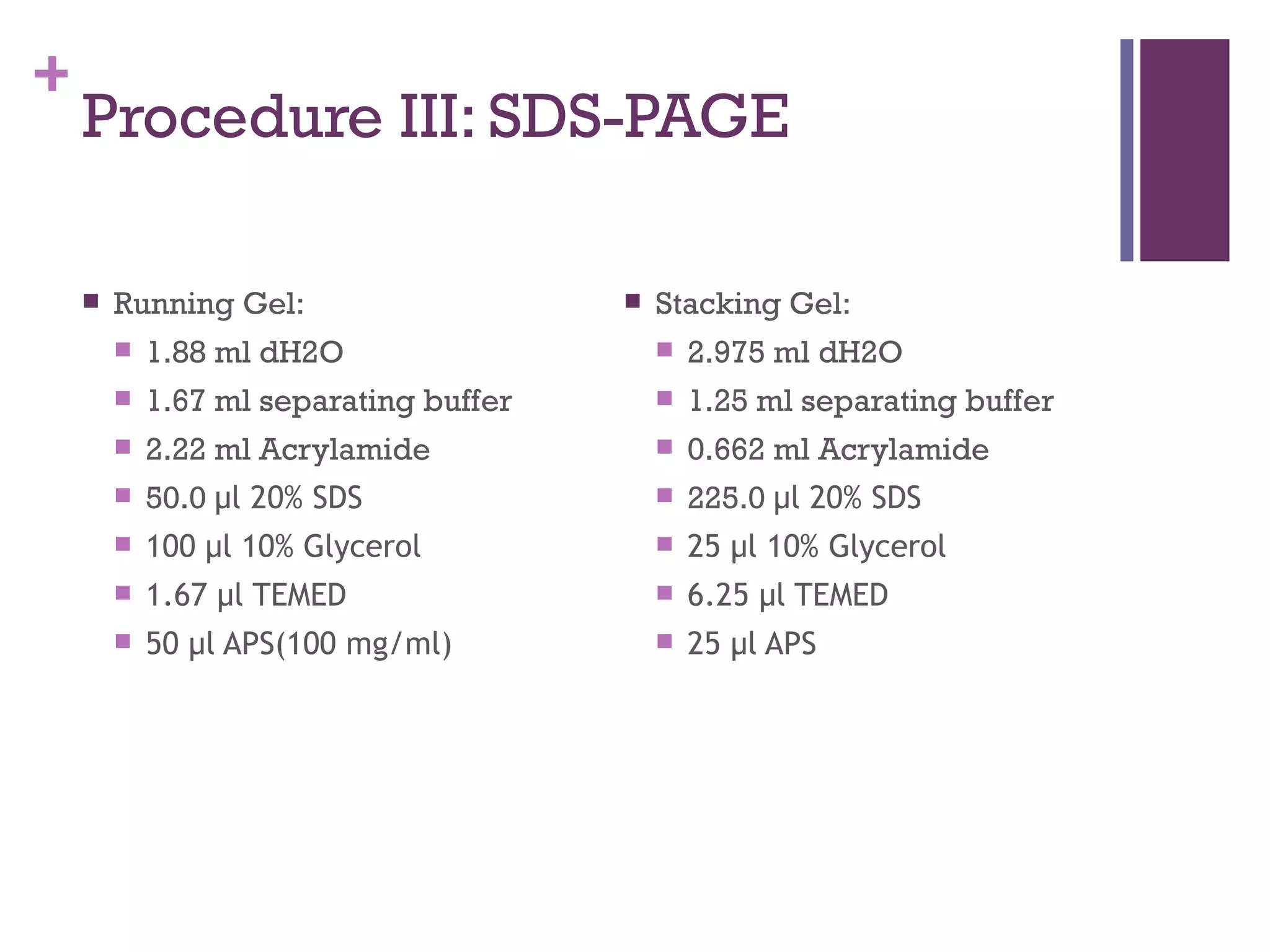
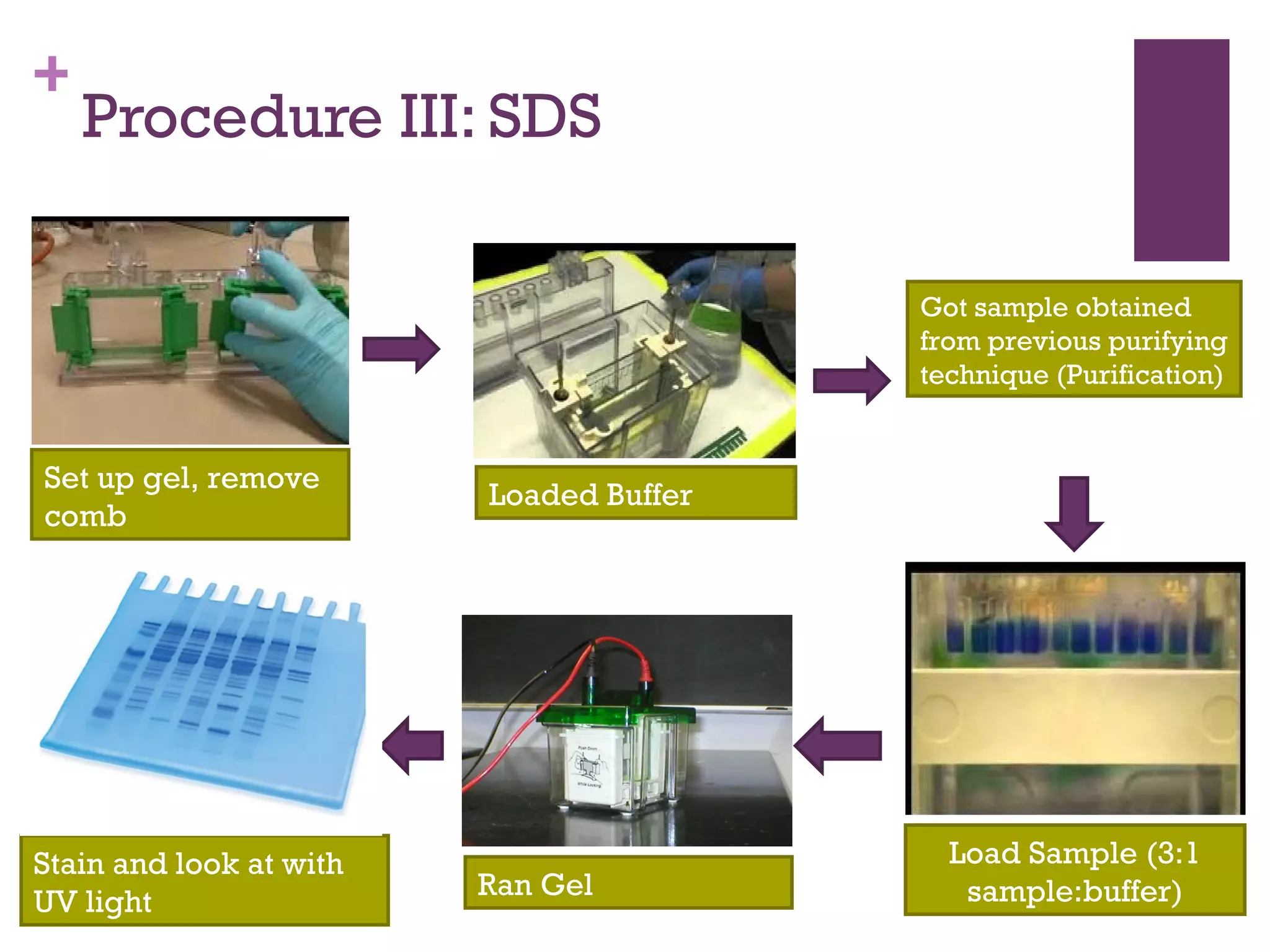
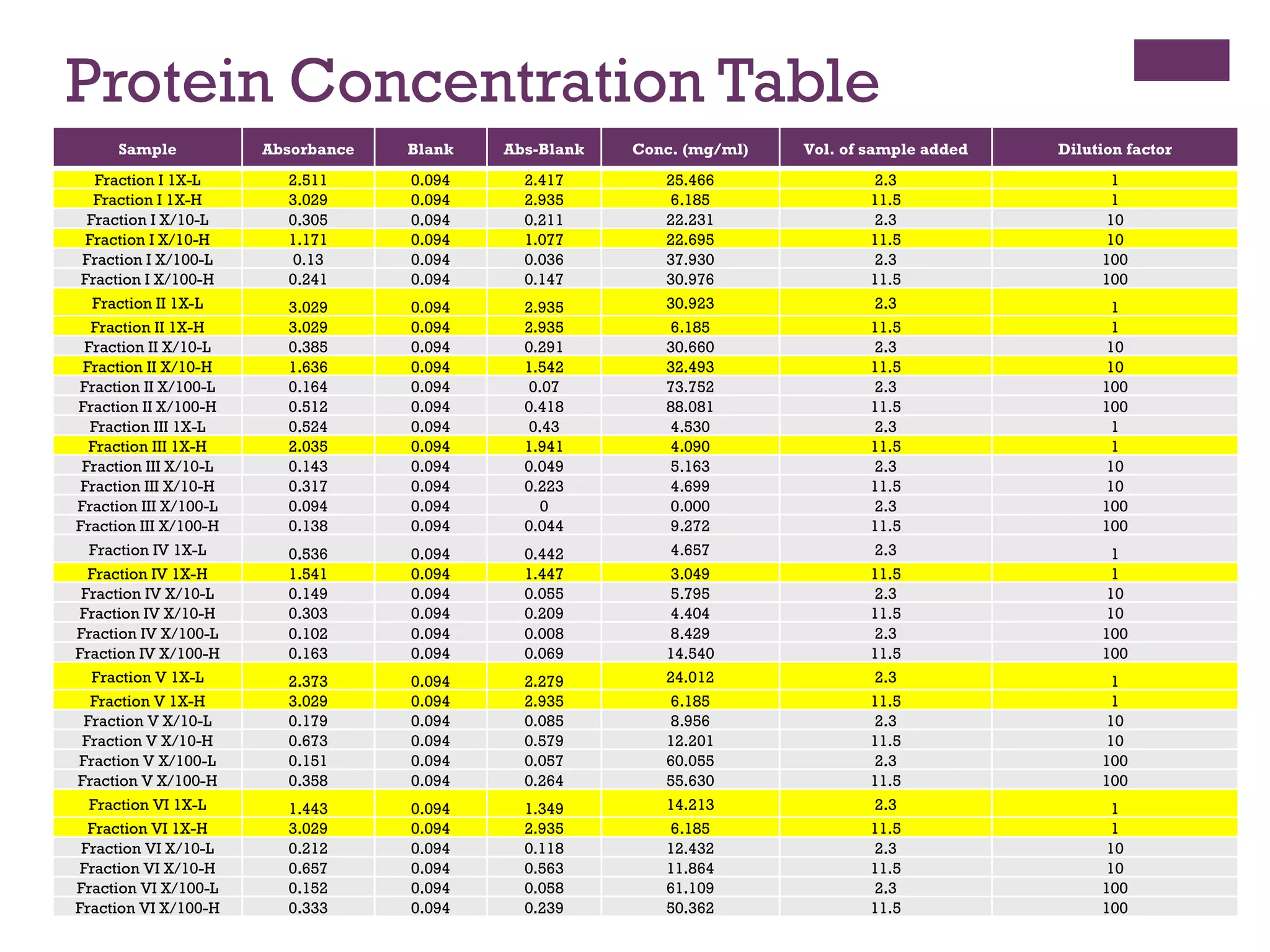
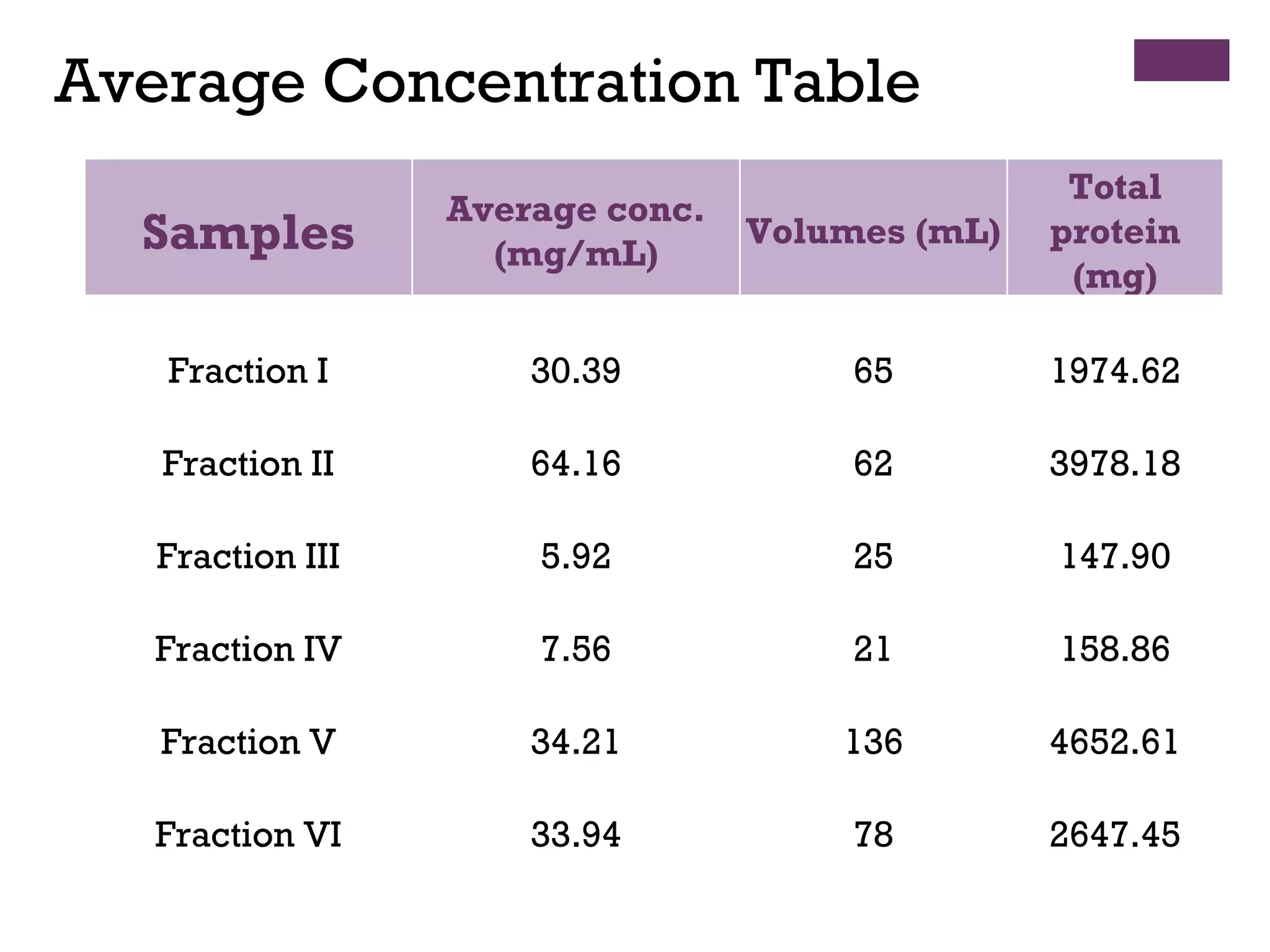
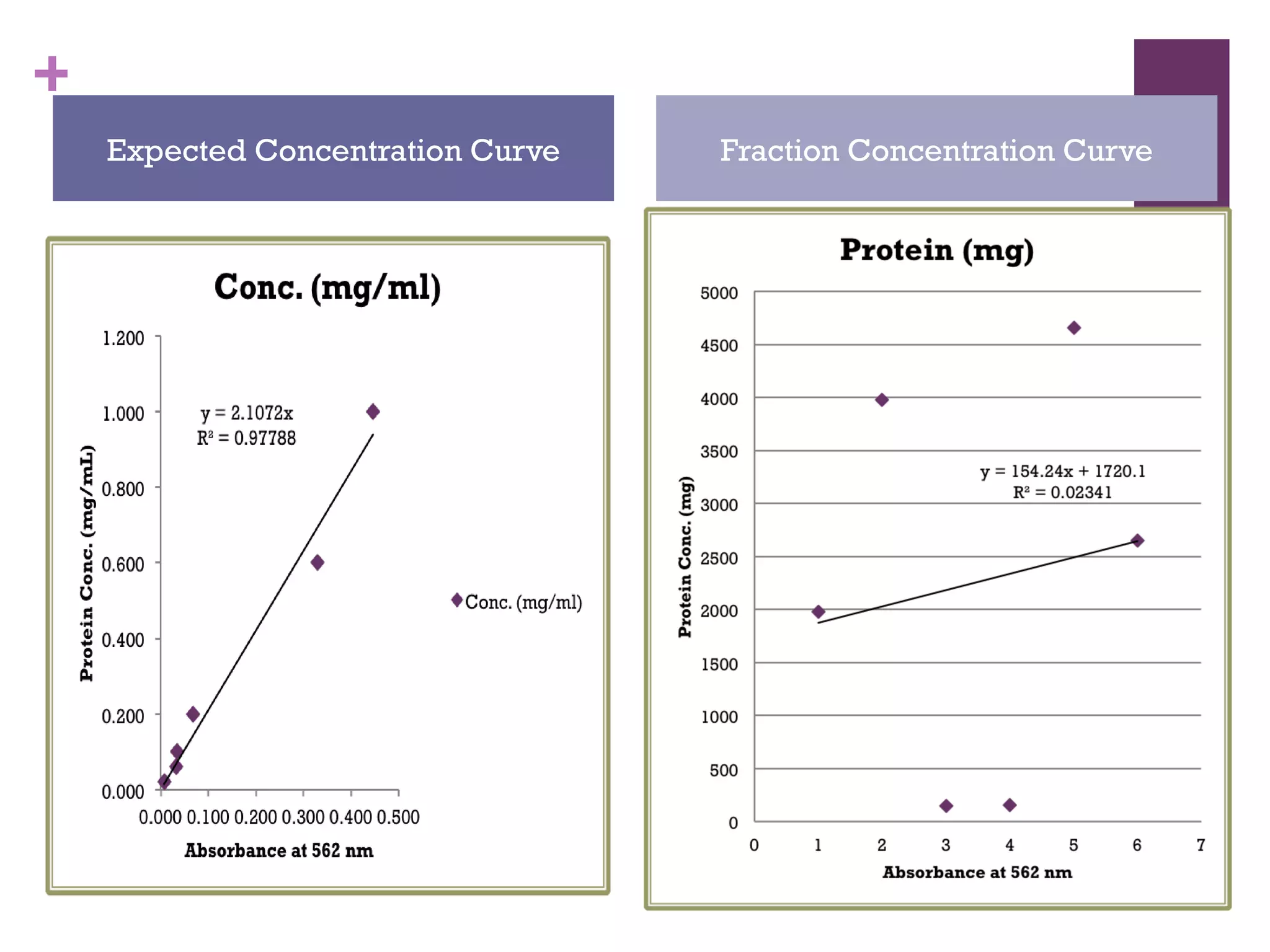
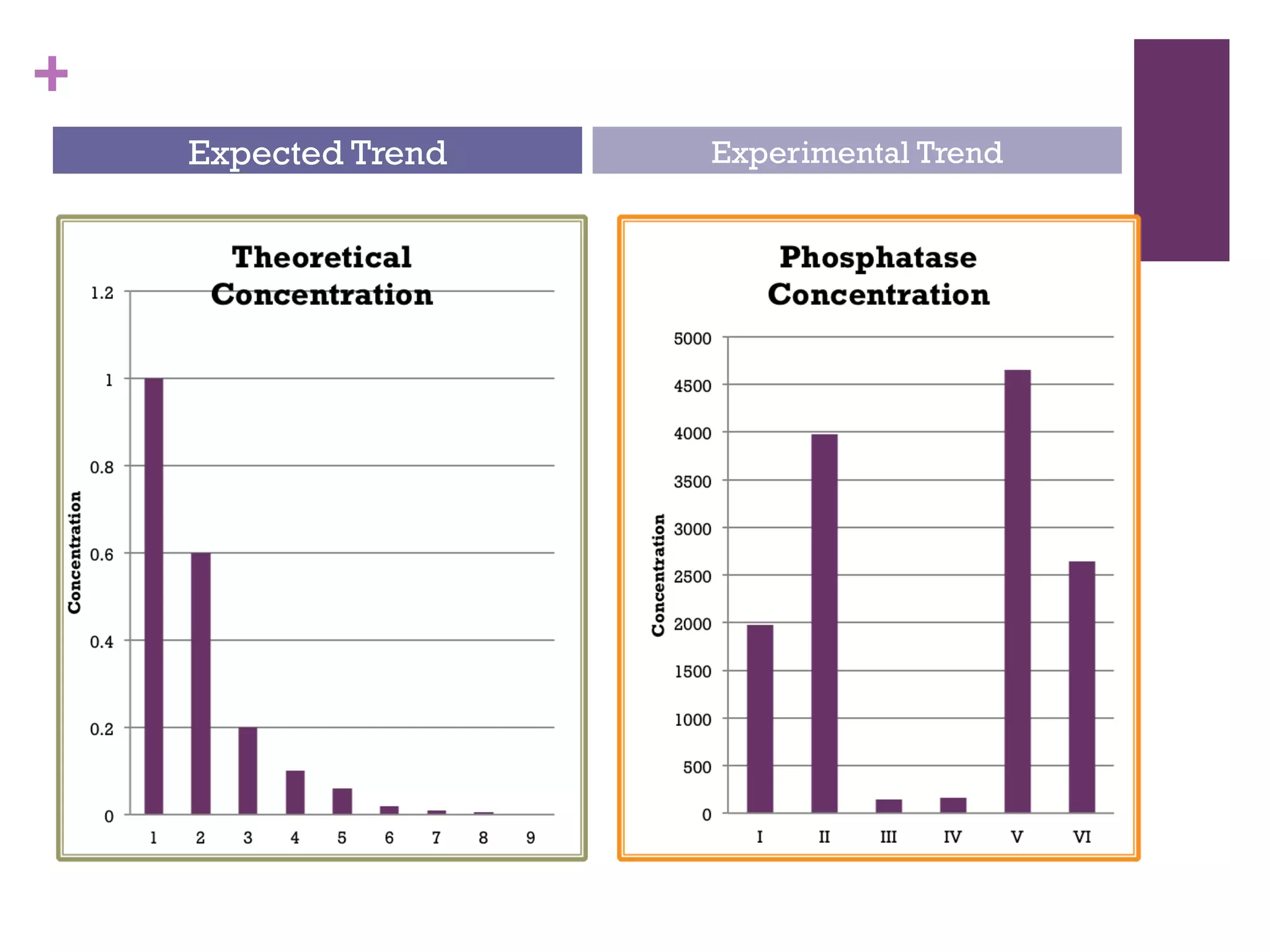
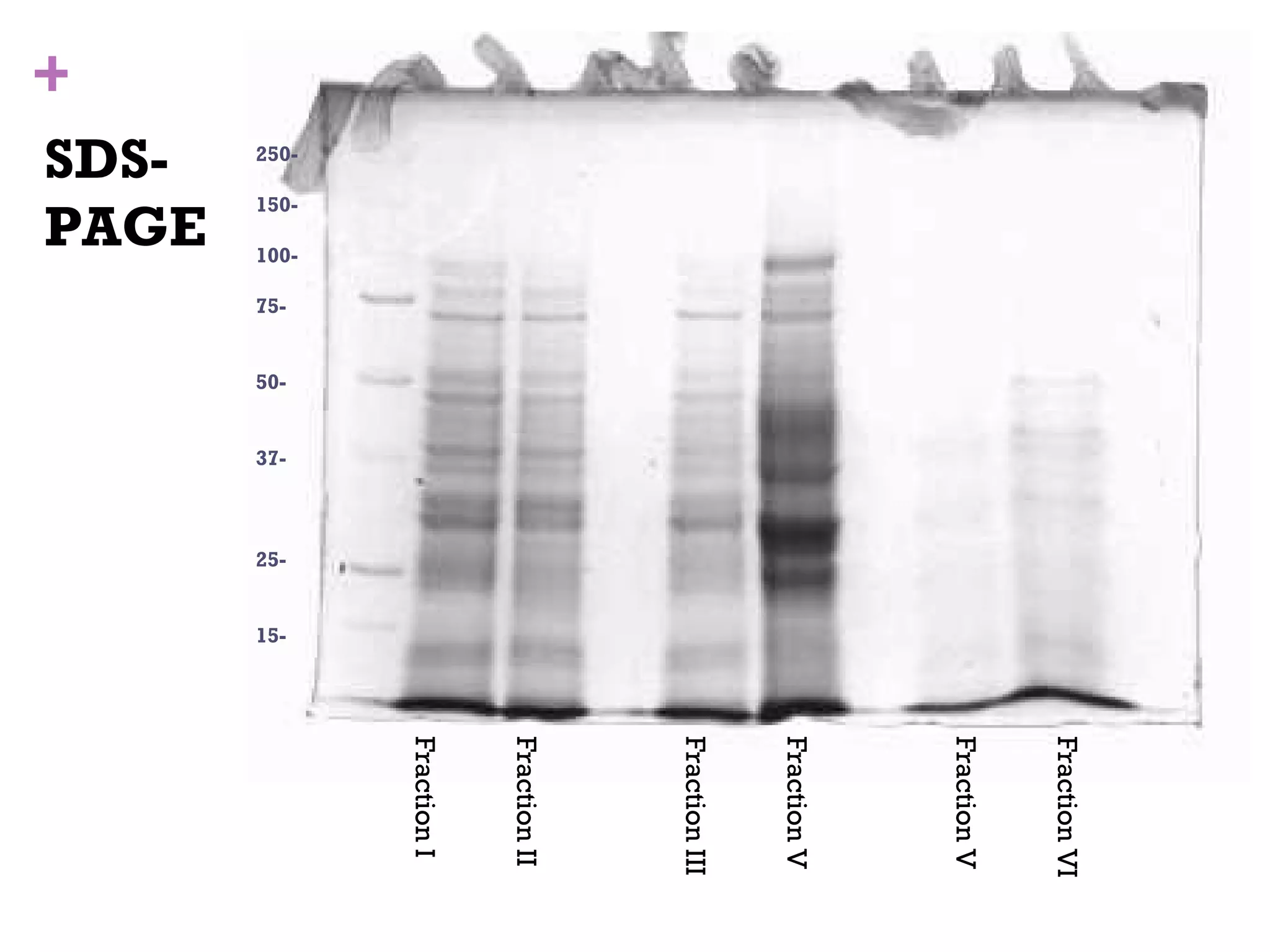
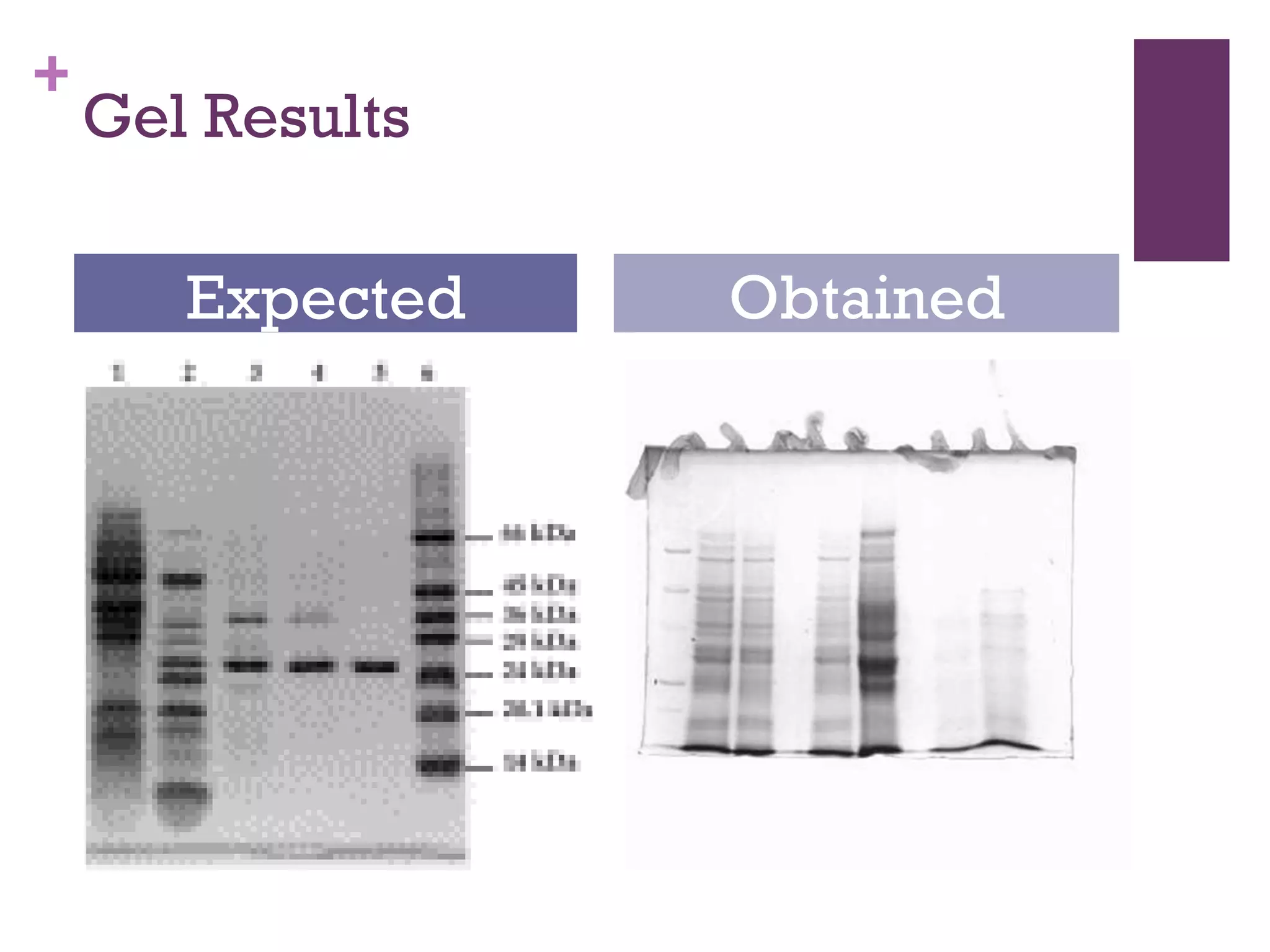
This document describes the isolation, purification, and assay of wheat germ acid phosphatase. The objectives were to purify acid phosphatase from wheat germ and determine its protein concentration. Wheat germ was homogenized and centrifuged to obtain 6 fractions. A BCA assay was used to measure the protein concentration of each fraction at different dilutions. SDS-PAGE was also performed to analyze the purified samples. The highest protein concentrations were found in Fractions I, II and V.









![+
References
1. Stoscheck ,2000. CM. Quantitation of Protein. Methods in
Enzymology, 50-69.
2. Yasuaki Kawarasaki, Hideo Nakano, Tsuneo Yamane, 1999.
Purification and some properties of wheat germ acid
phosphatases. Elsvier
[http://www.sciencedirect.com/science/article/pii/016894529604
4779]
3. Ke-Xue Zhu,Hui-Ming Zhou, and Hai-Feng Qian, 2008. Proteins
Extracted from Defatted Wheat Germ: Nutritional and Structural
Properties
[http://cerealchemistry.aaccnet.org/doi/abs/10.1094/CC-83-0069
]
4. VERJEE Z. H. M., 1969. Isolation of Three Acid Phosphatases from
Wheat Germ. European J. Biochem., 439-44.](https://image.slidesharecdn.com/wilmarieandnataliaproteinisolation-120521205154-phpapp01/75/adfb-oadf-24-2048.jpg)
