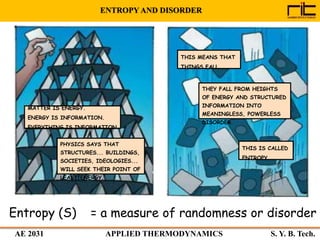
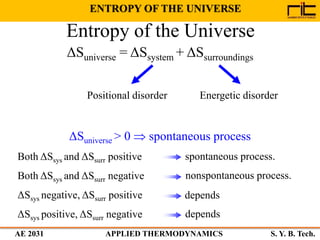
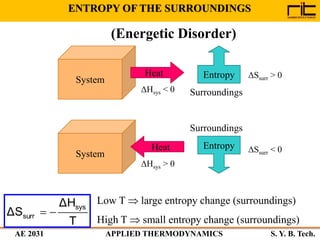
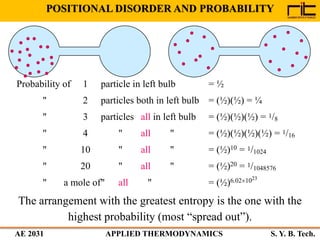
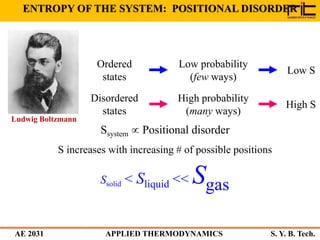
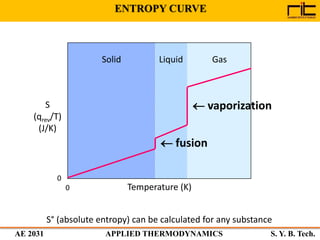
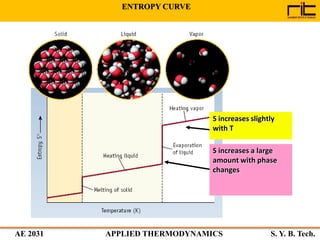
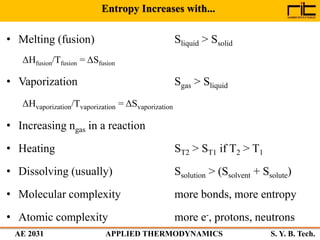
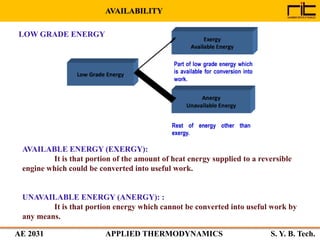
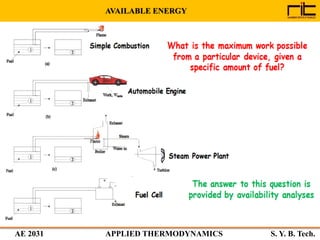
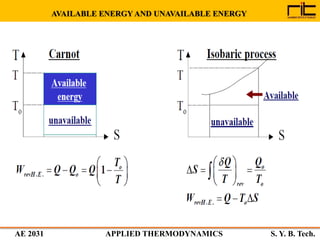
The document provides an outline and overview of the key concepts related to entropy. It begins by defining entropy as a measure of randomness or disorder and outlines Clausius' inequality and the increase of entropy principle as it relates to the second law of thermodynamics. It then discusses how entropy applies to systems and substances, both closed and open systems, and how entropy changes with phase changes, temperature changes, and other processes. The document provides examples and explanations of concepts like positional disorder, entropy curves, and sources of increased entropy.