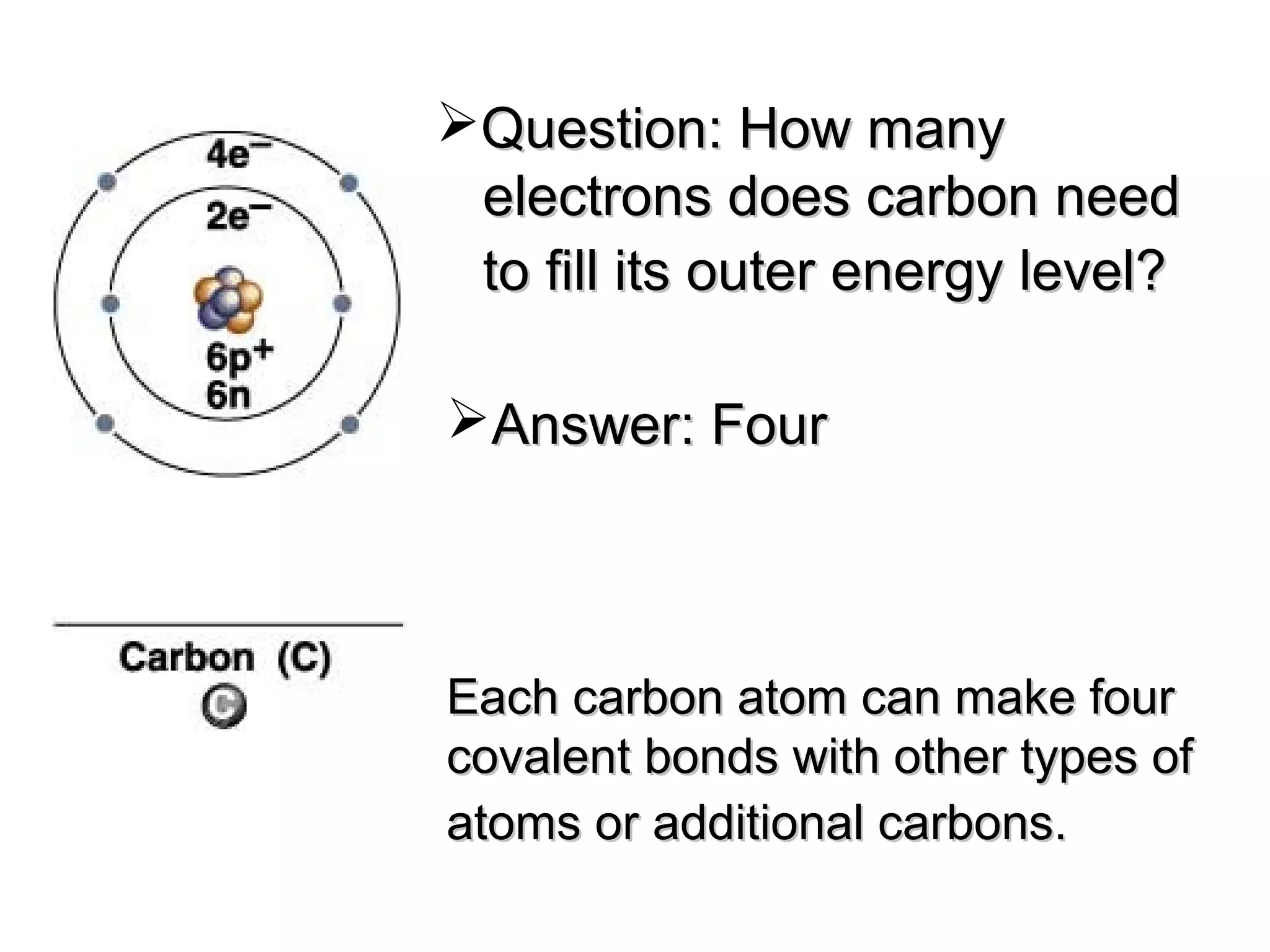
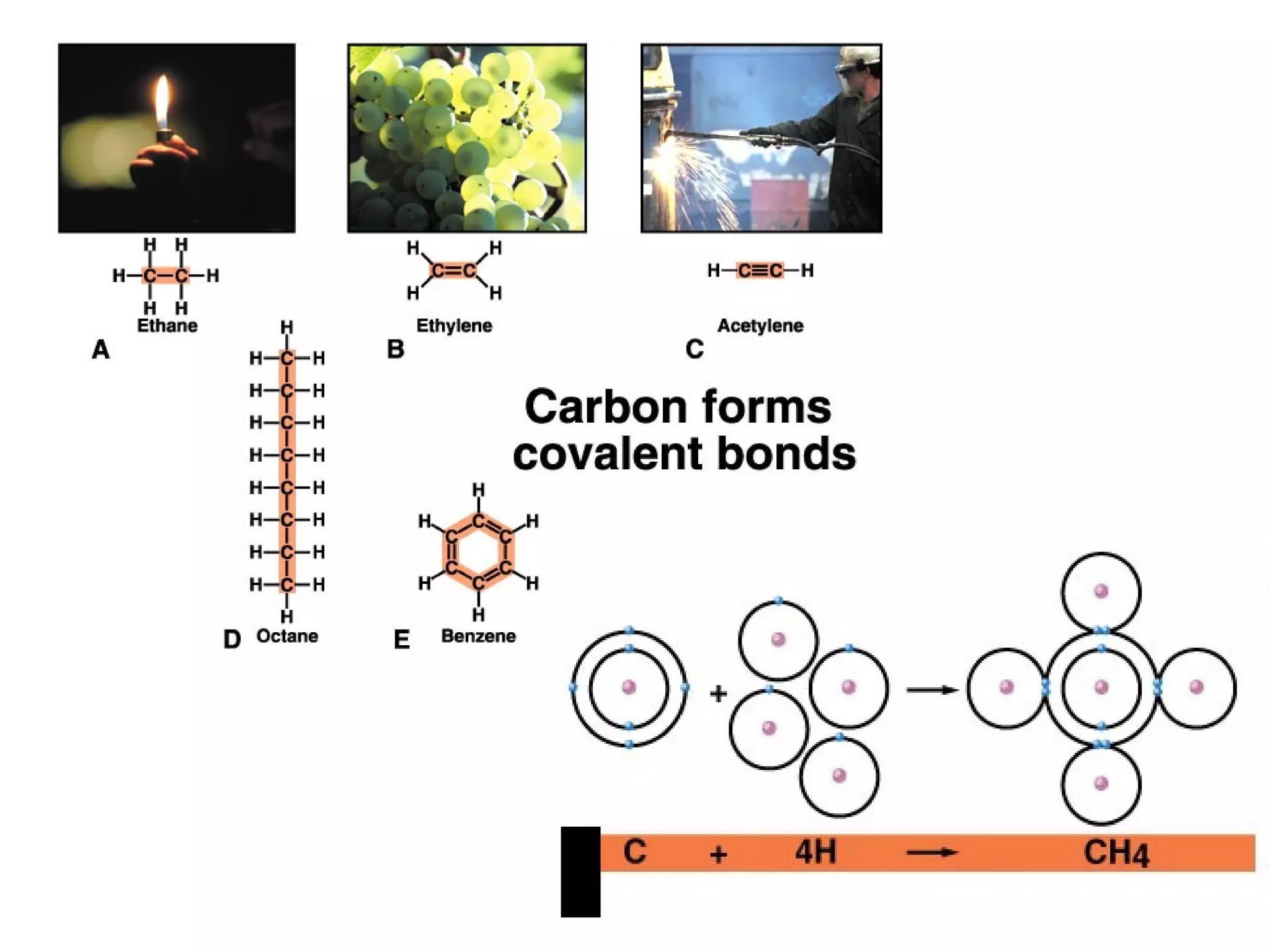
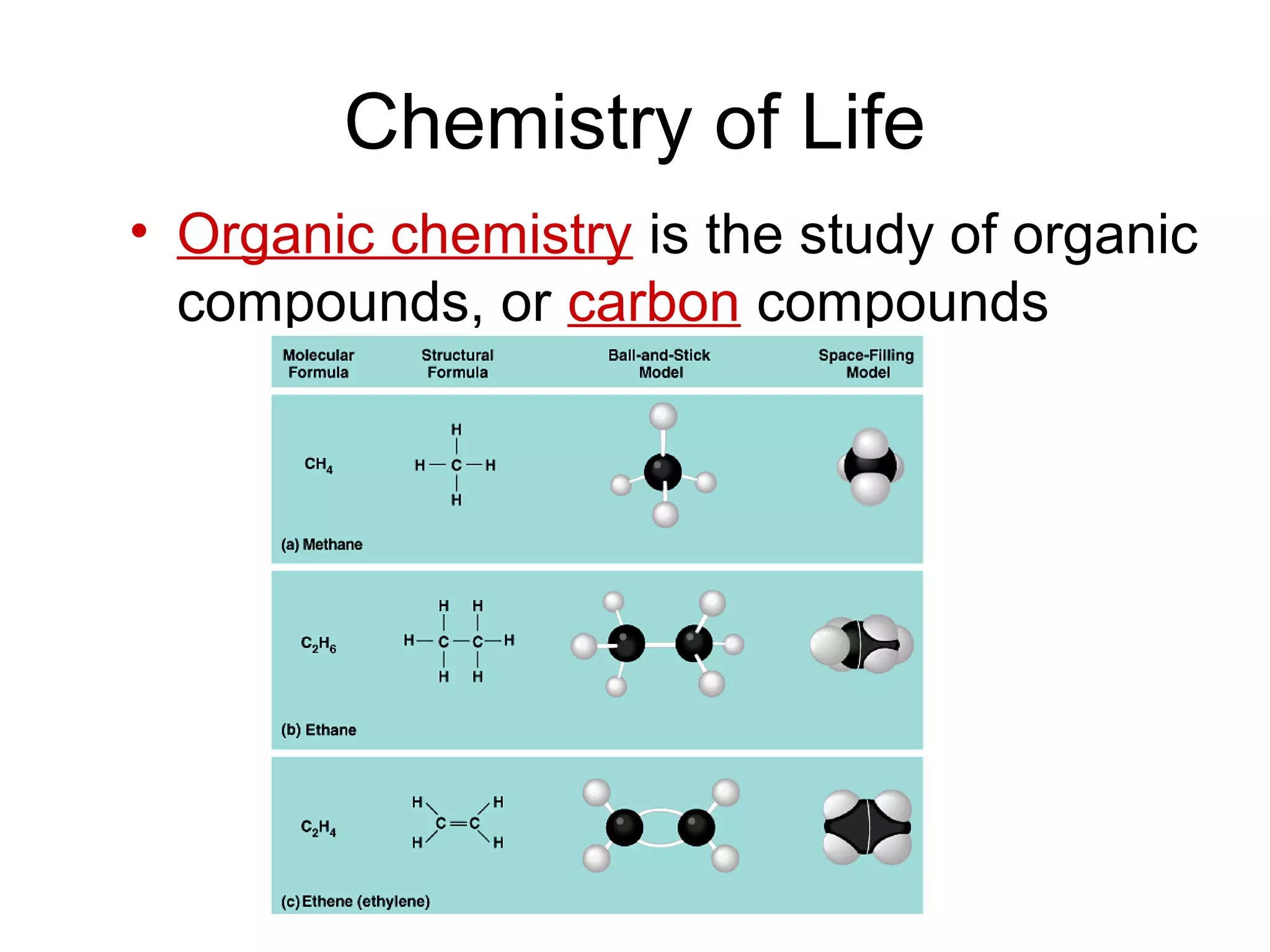
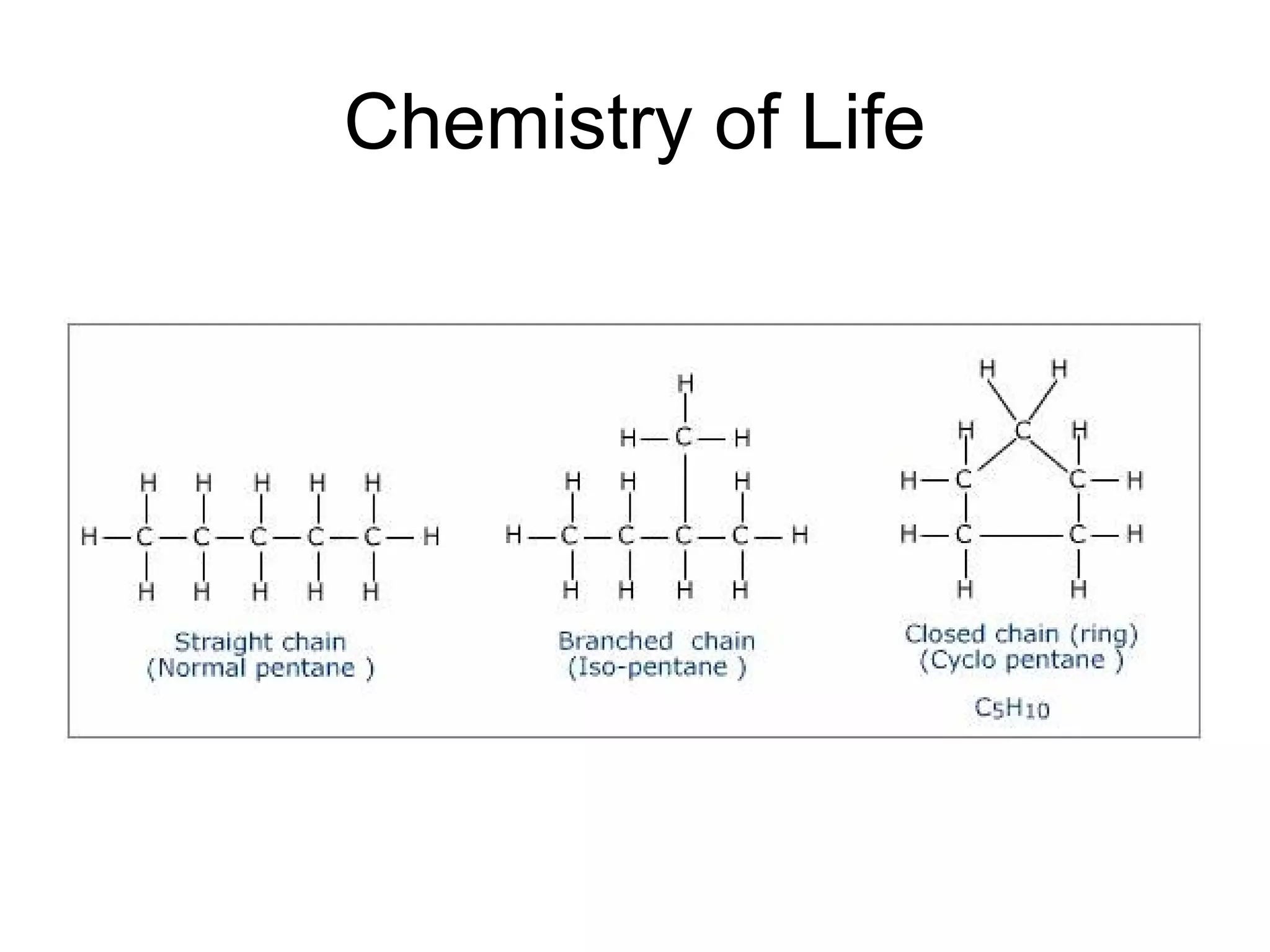
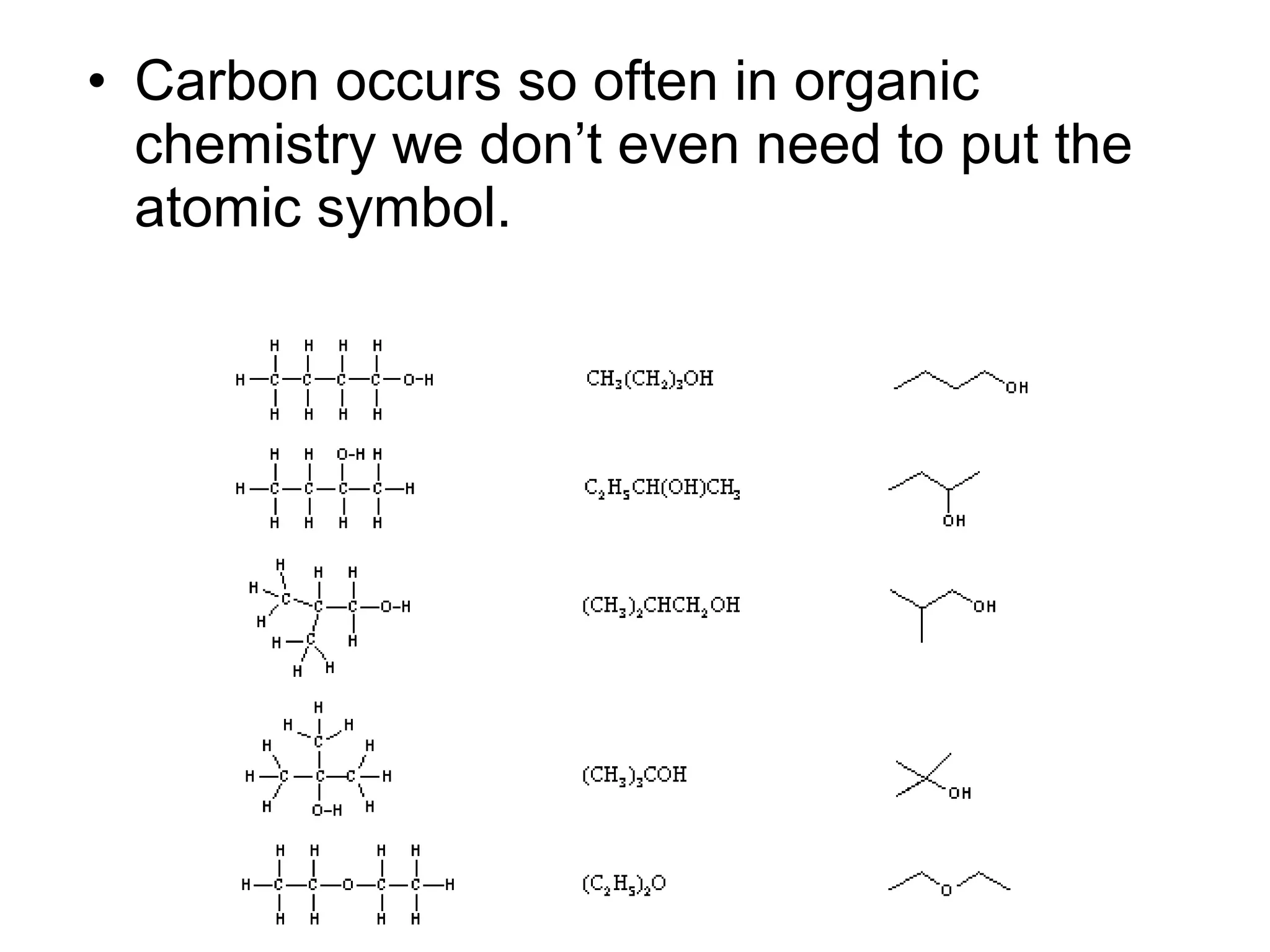
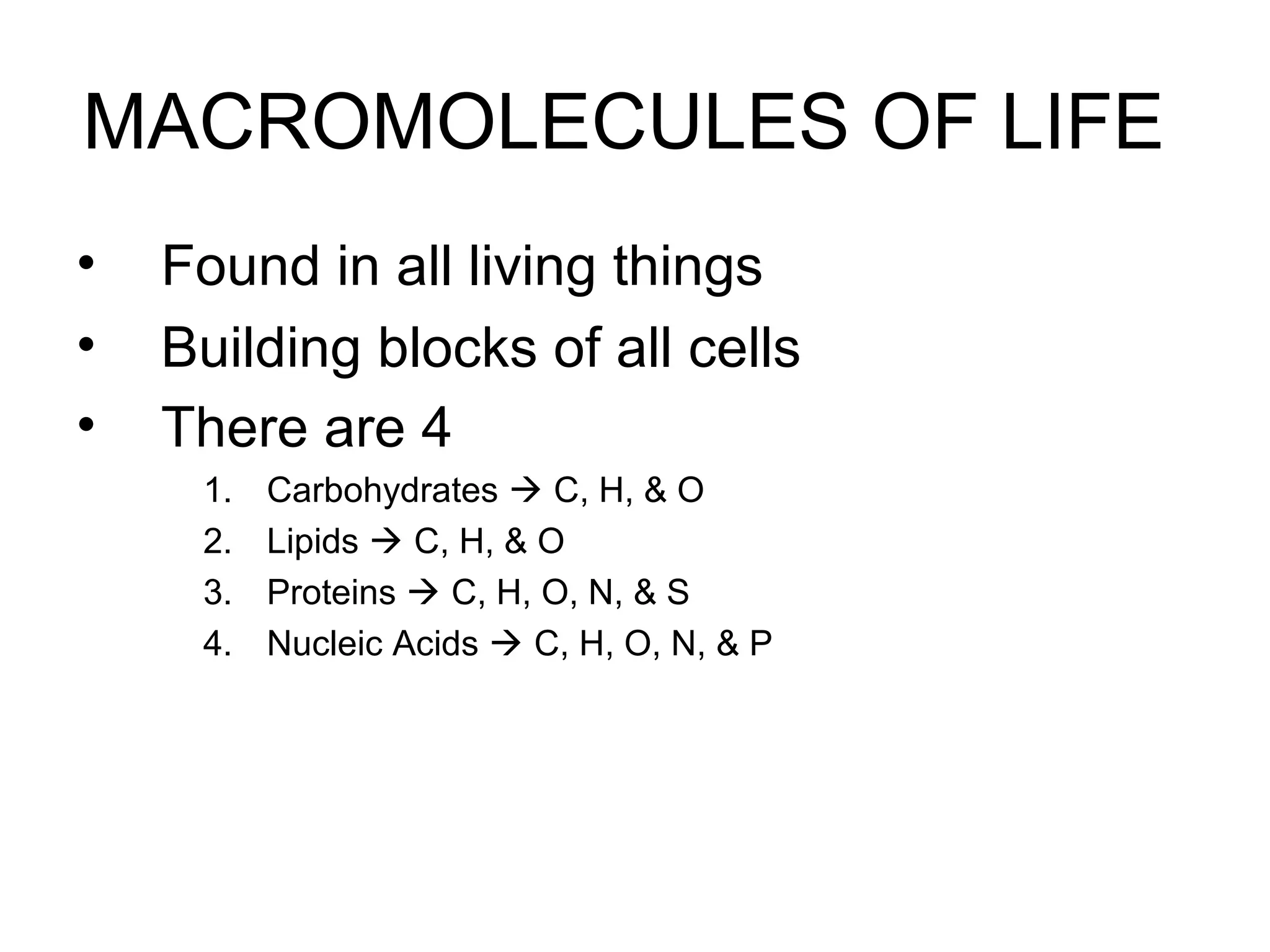
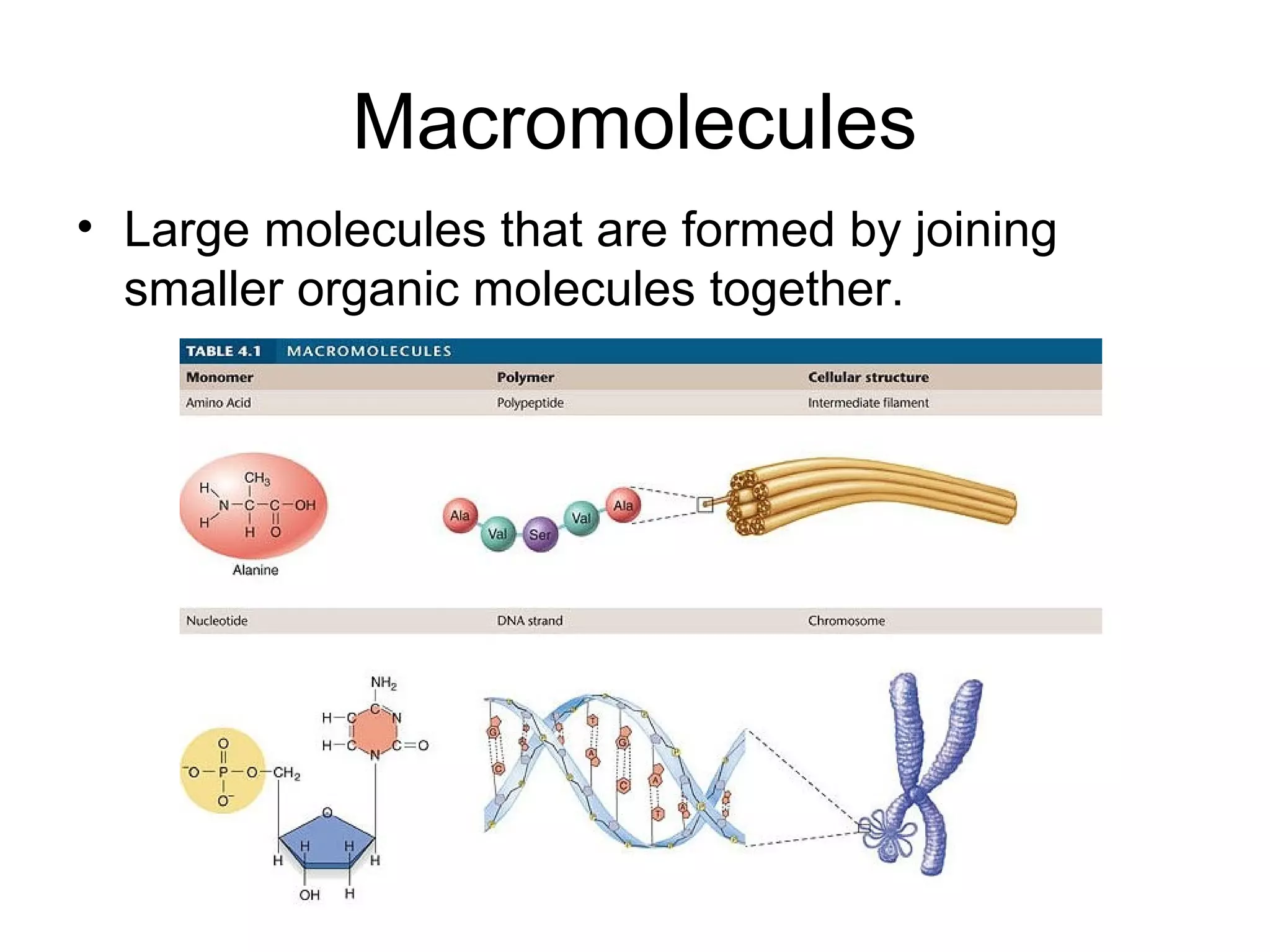
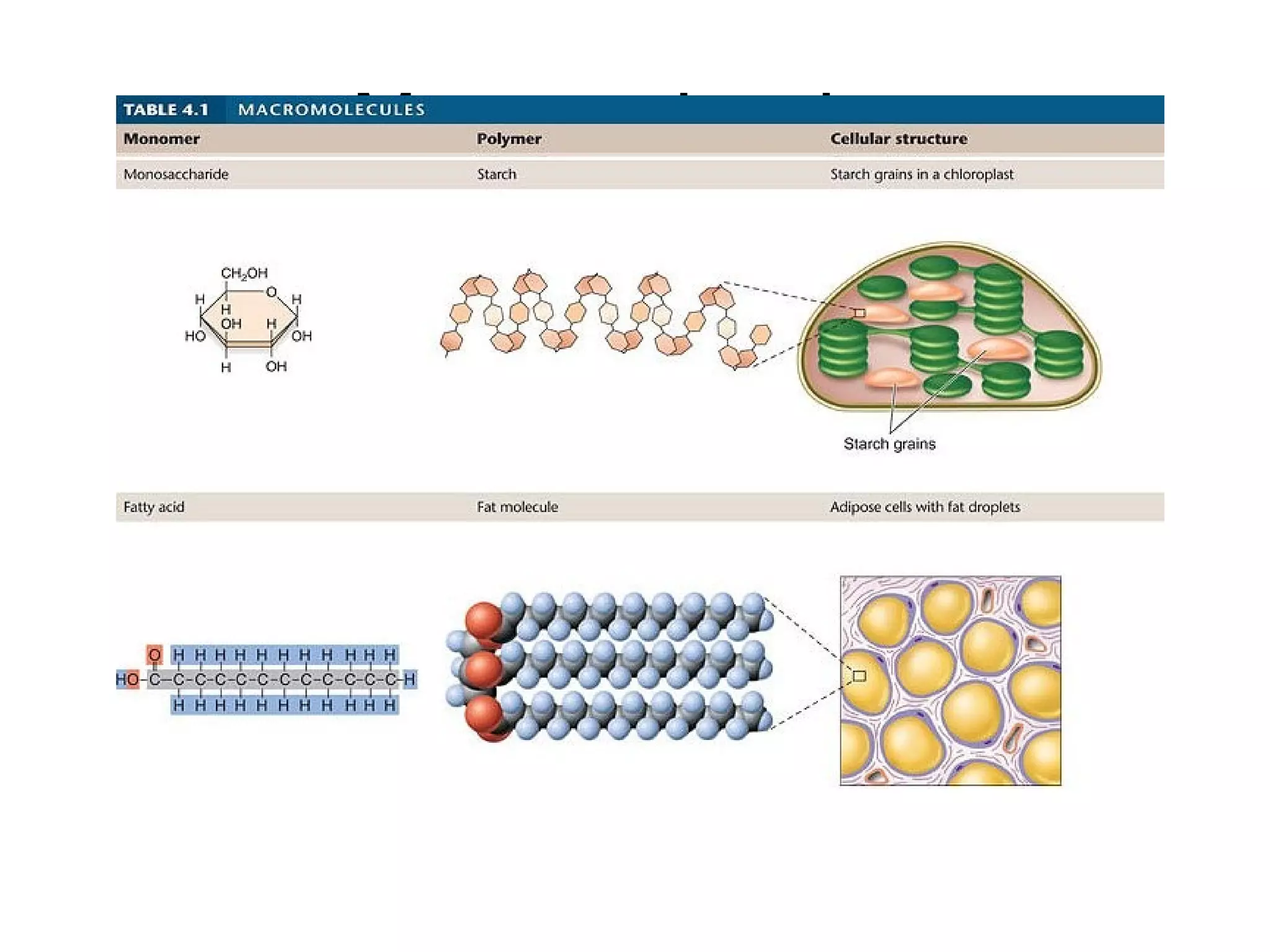
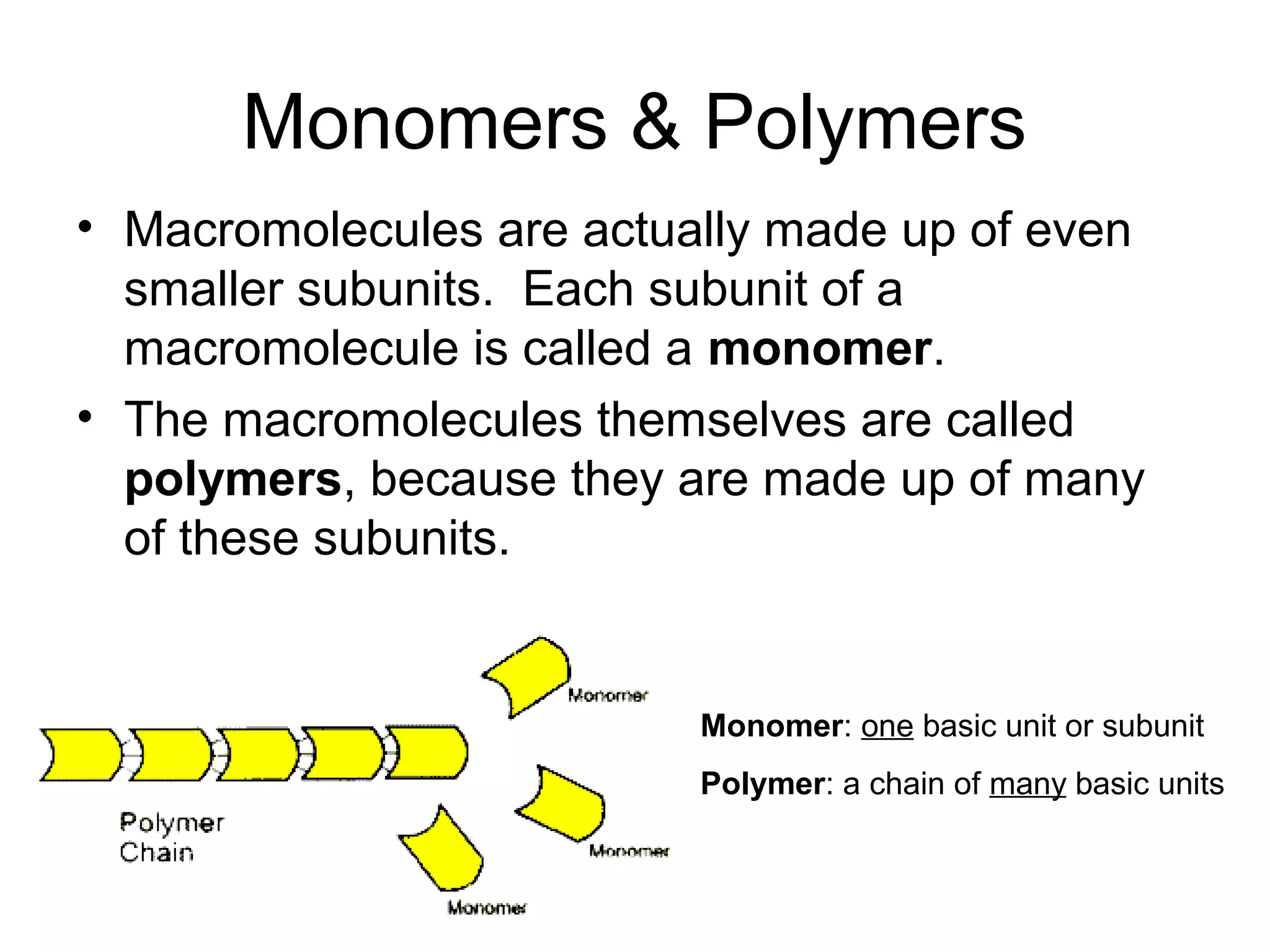
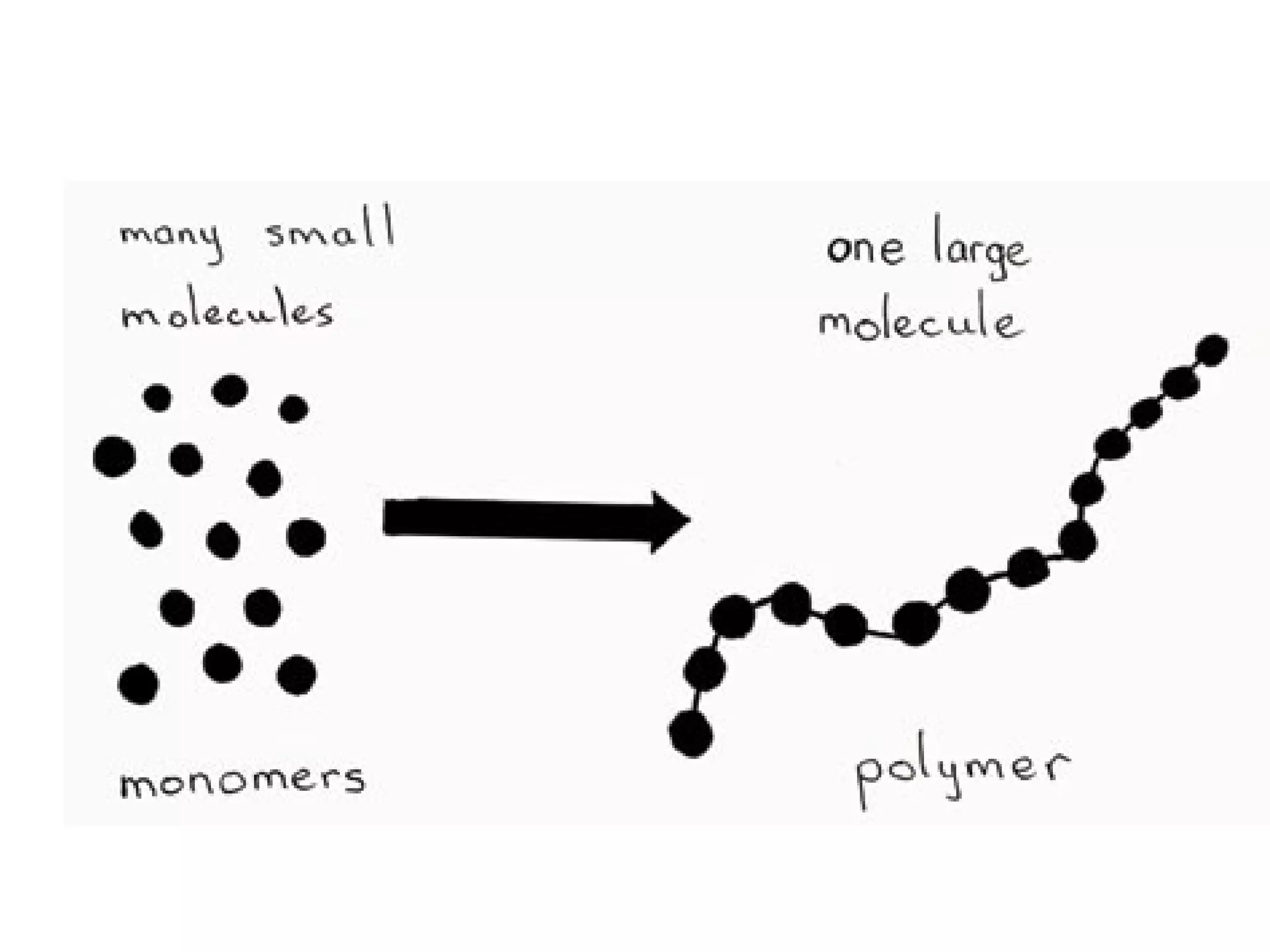
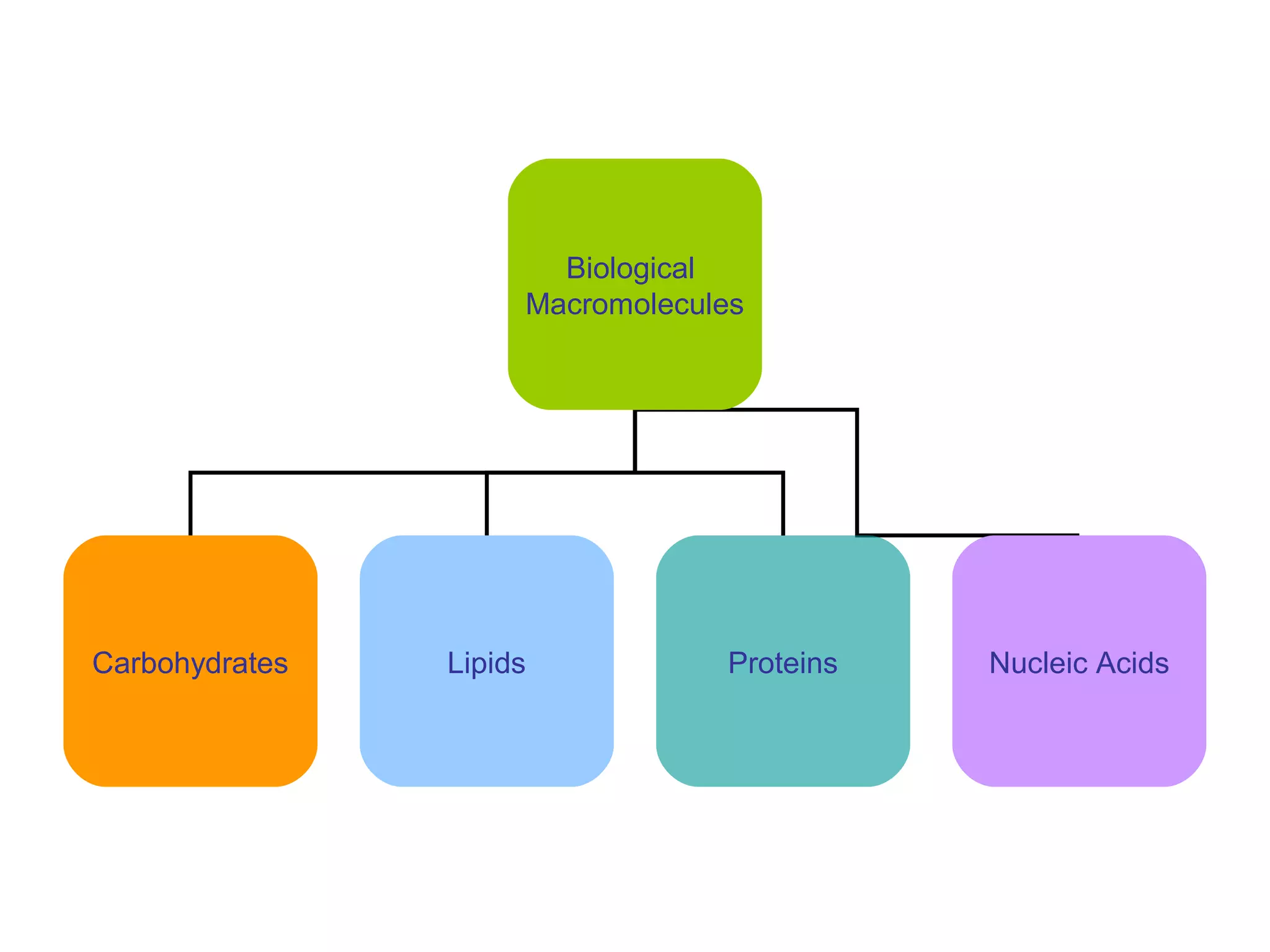
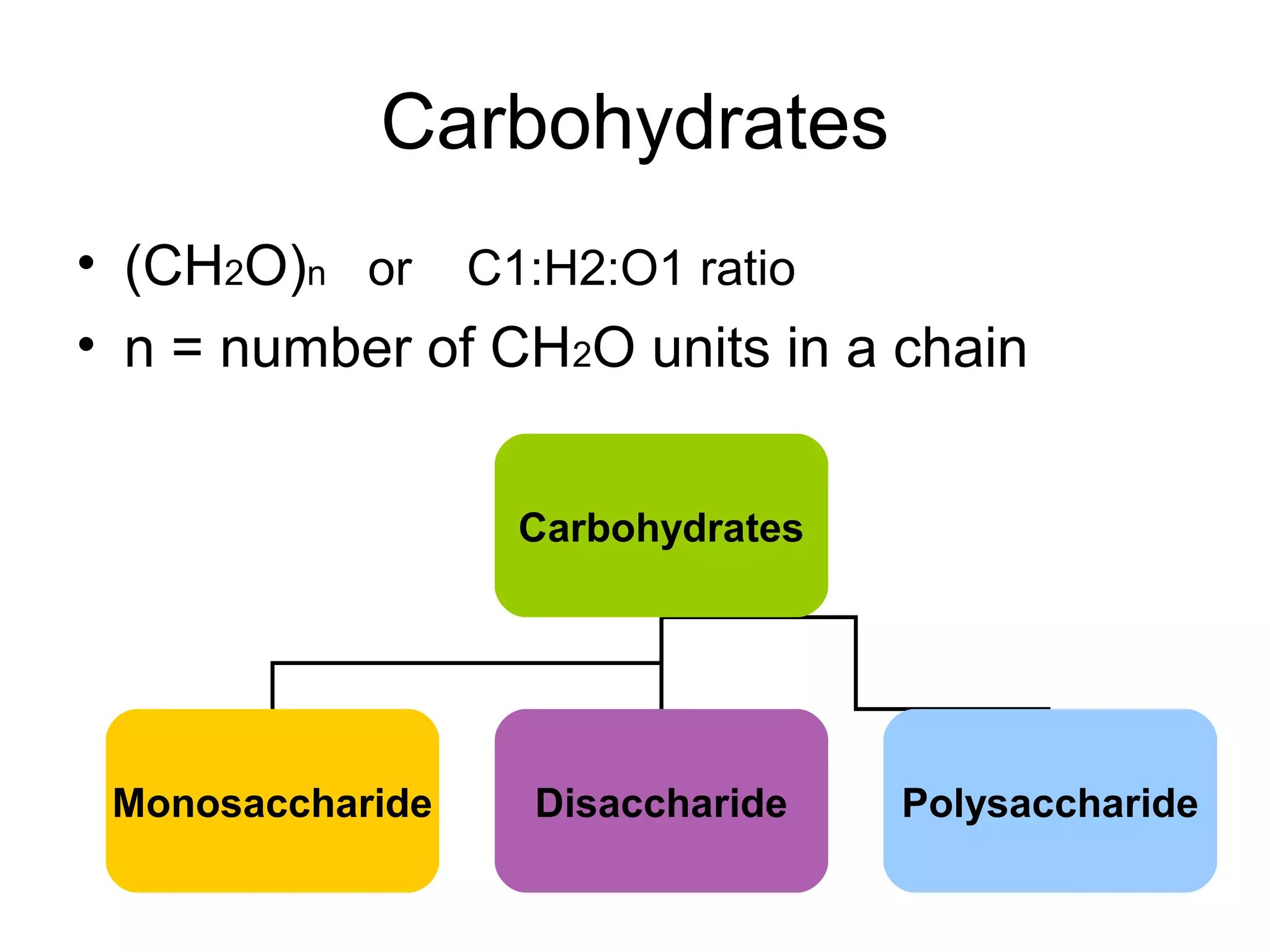
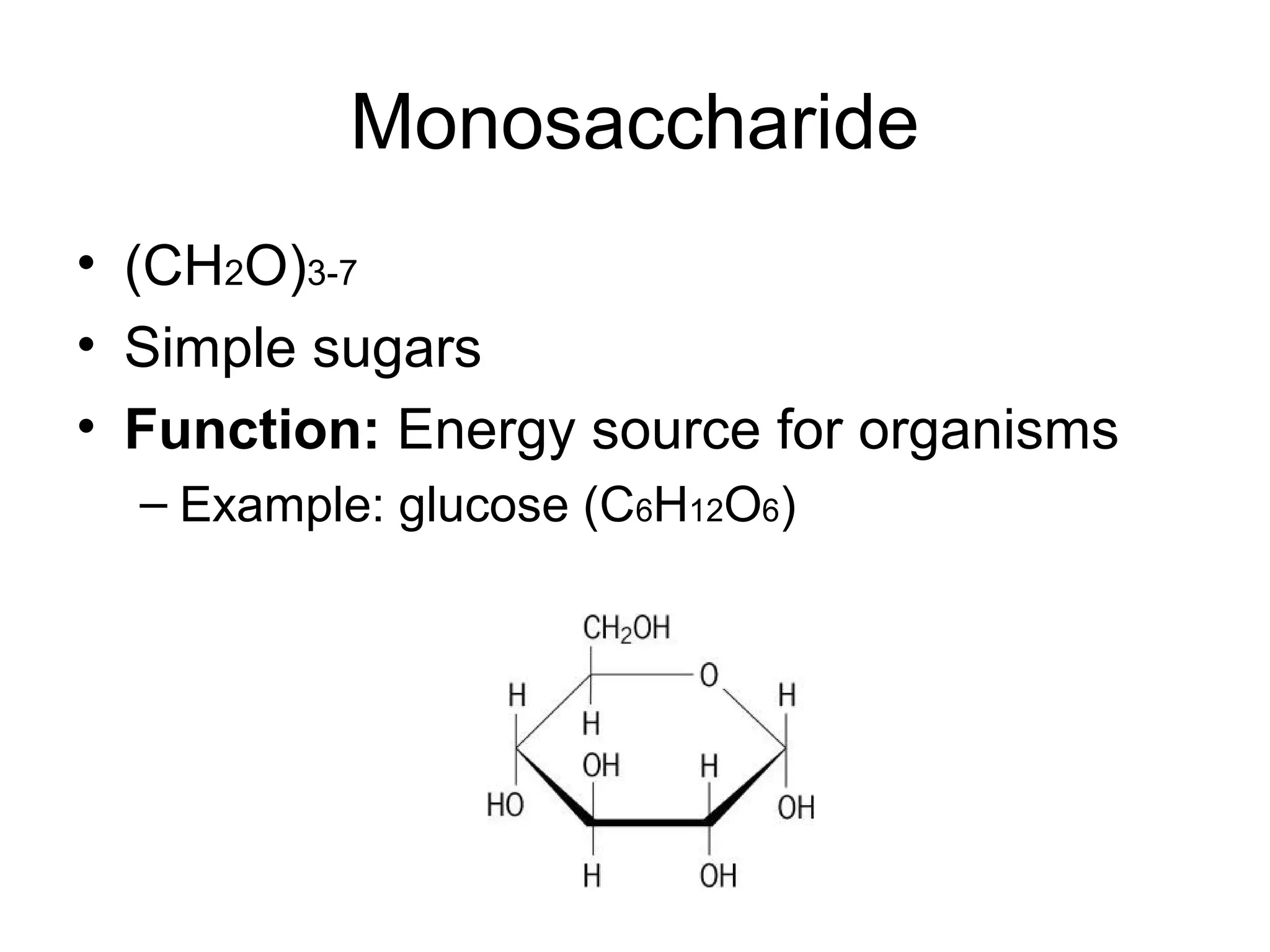
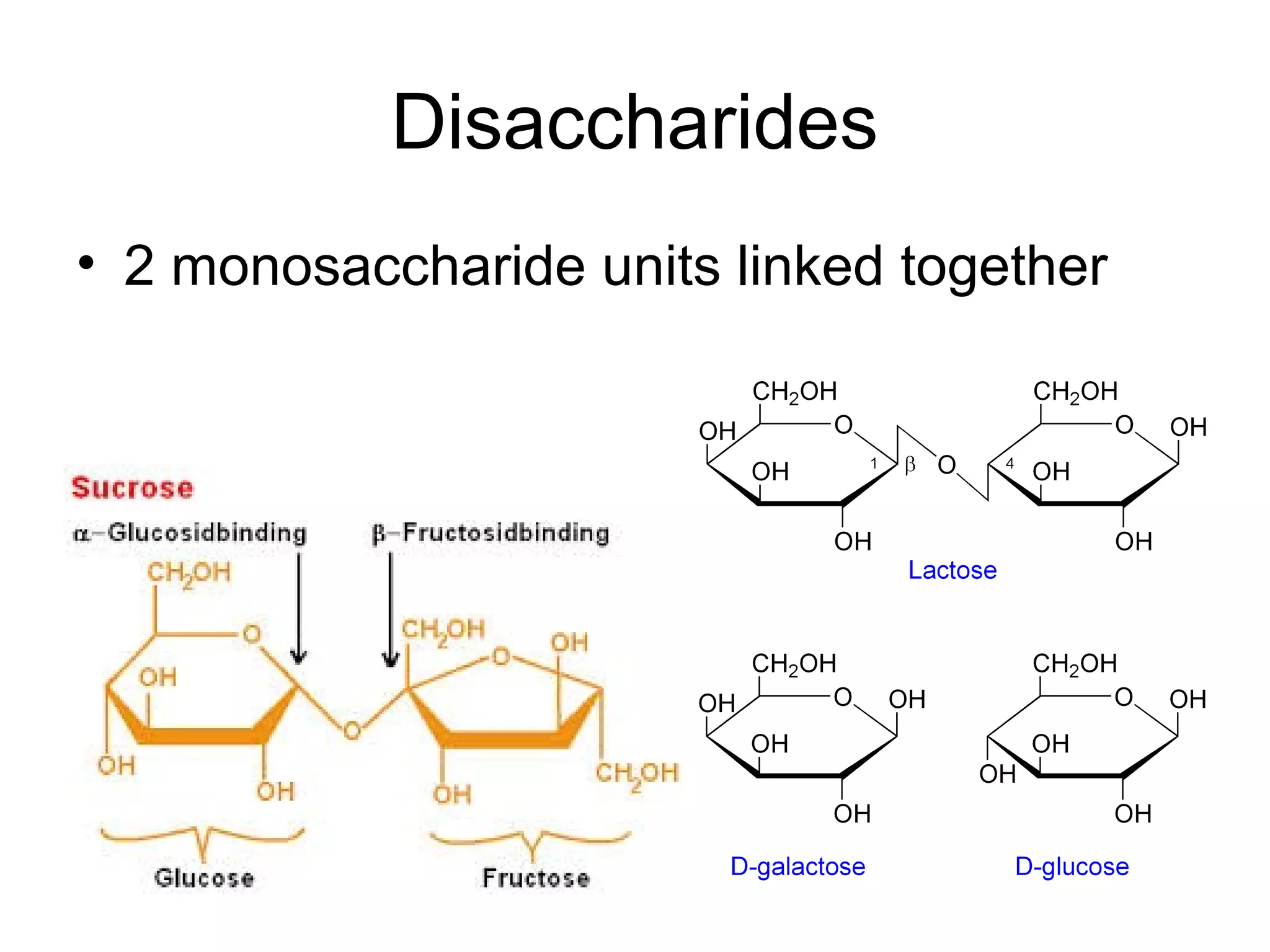
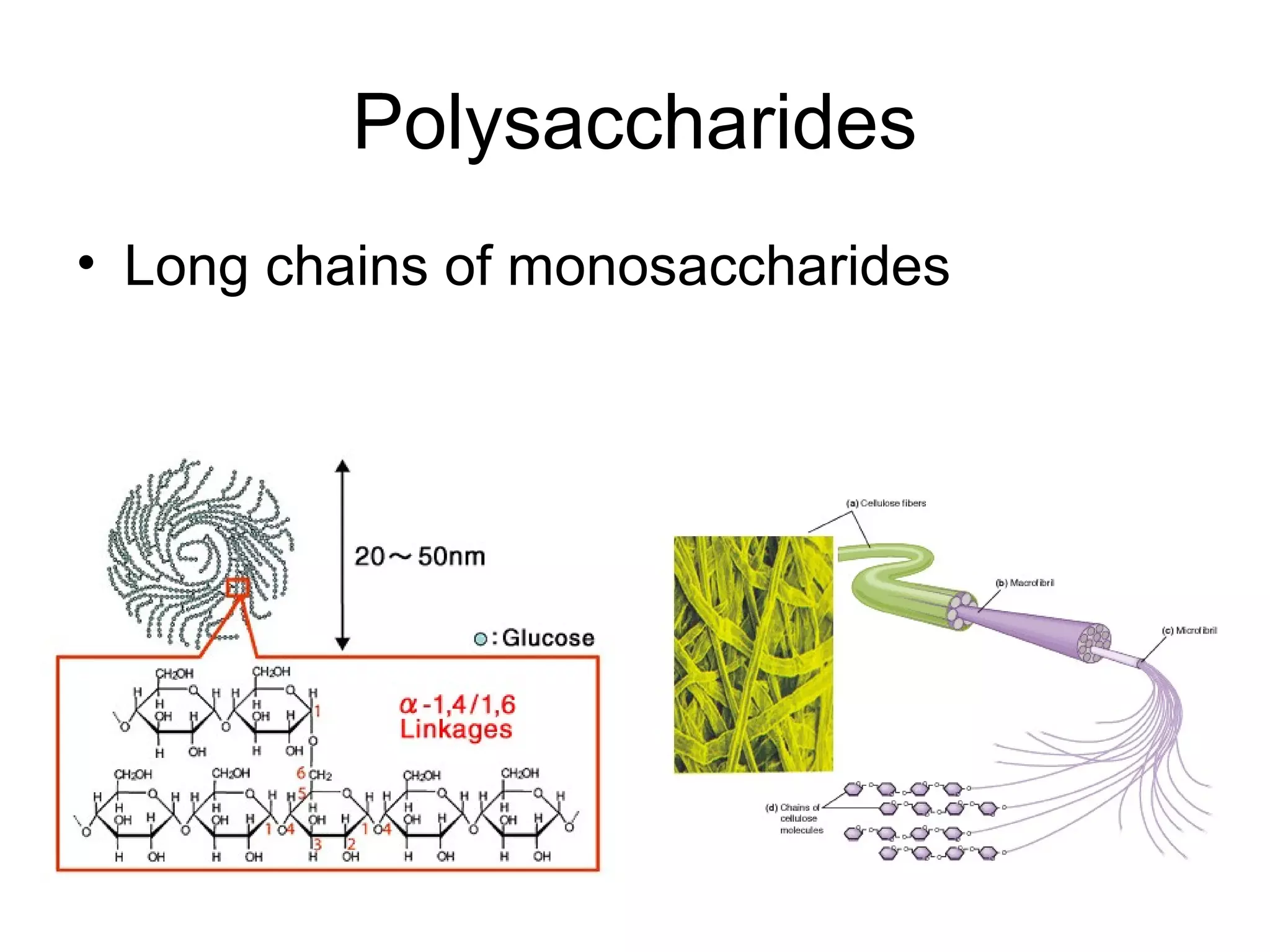
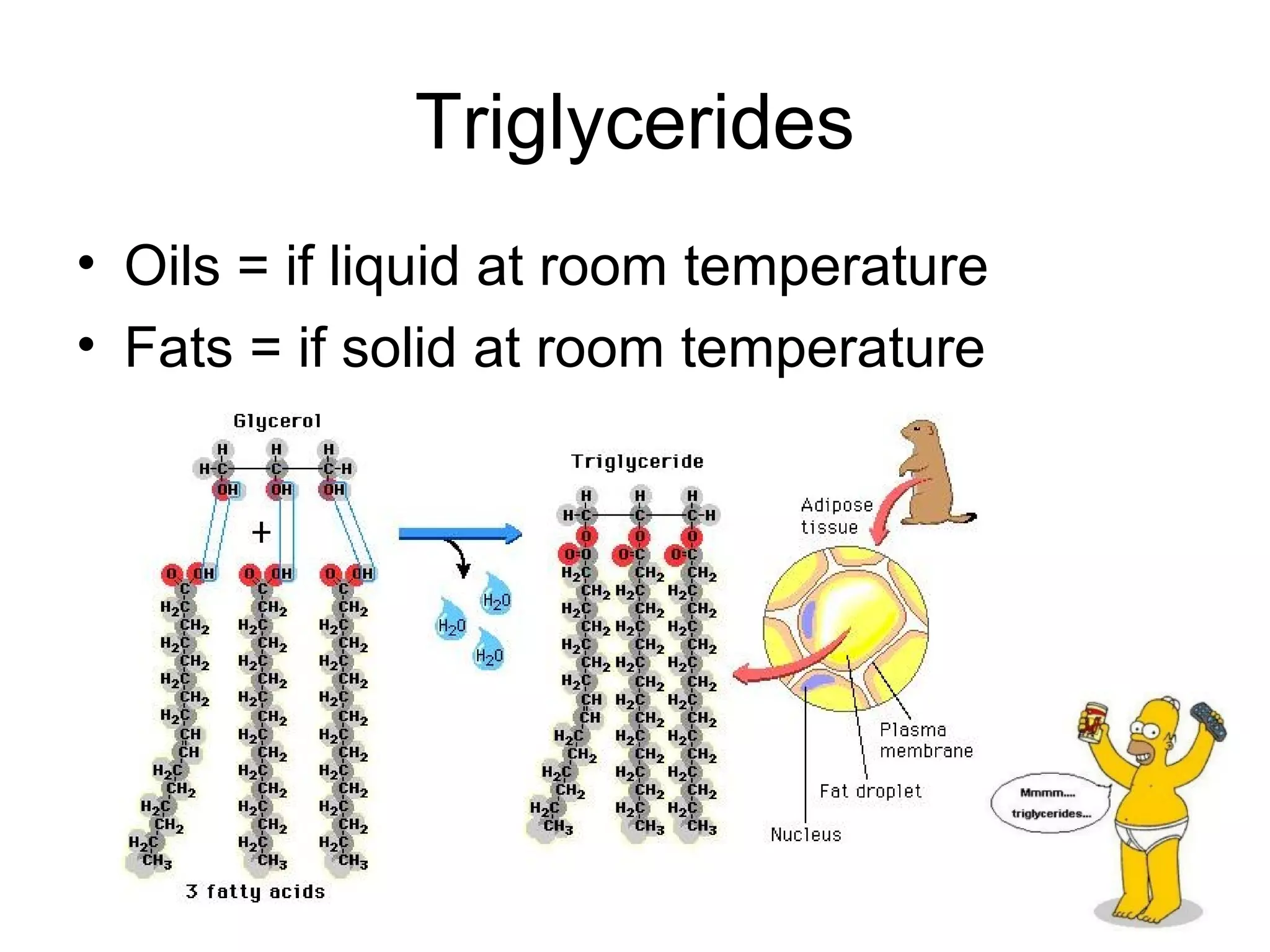
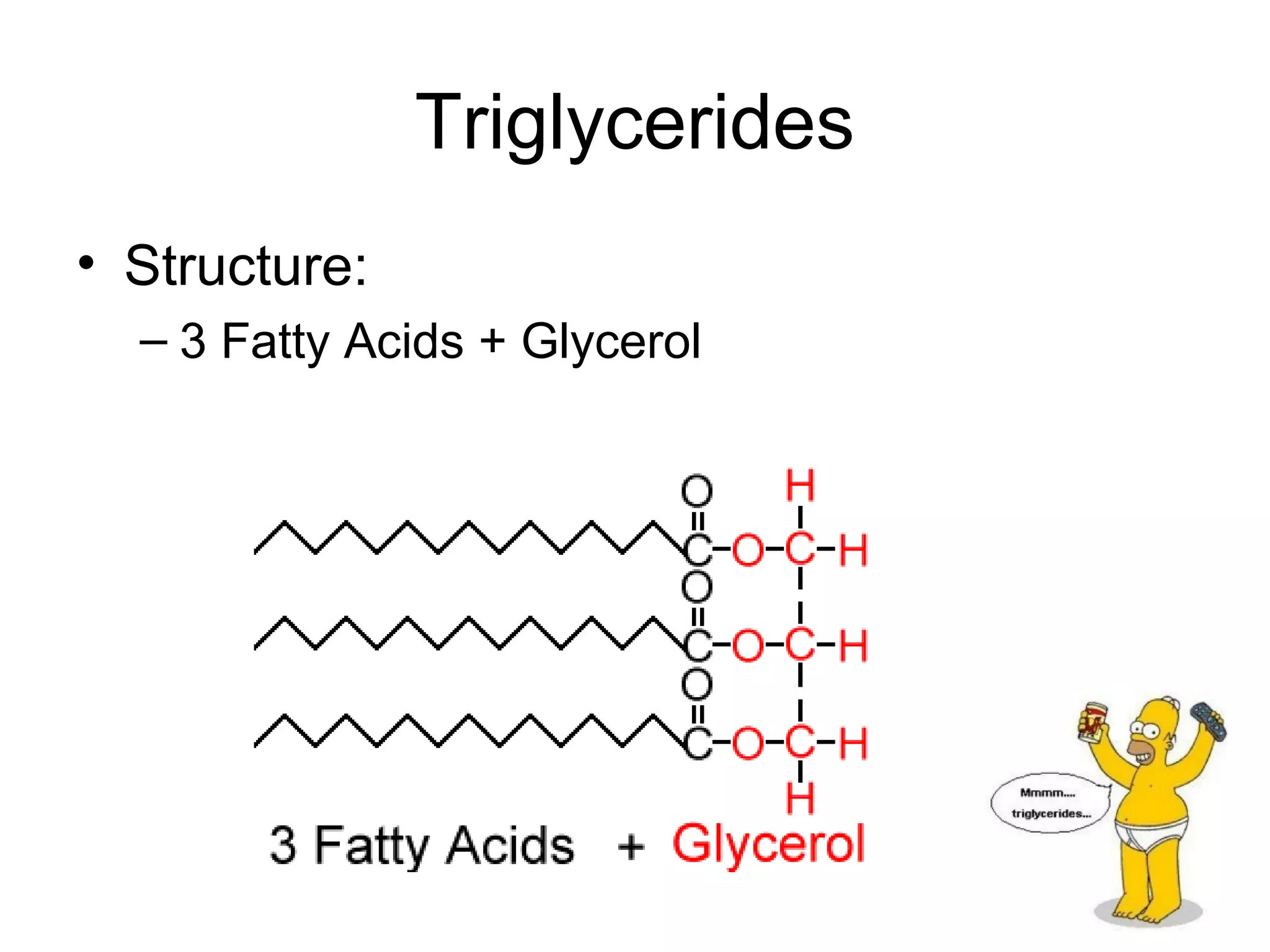
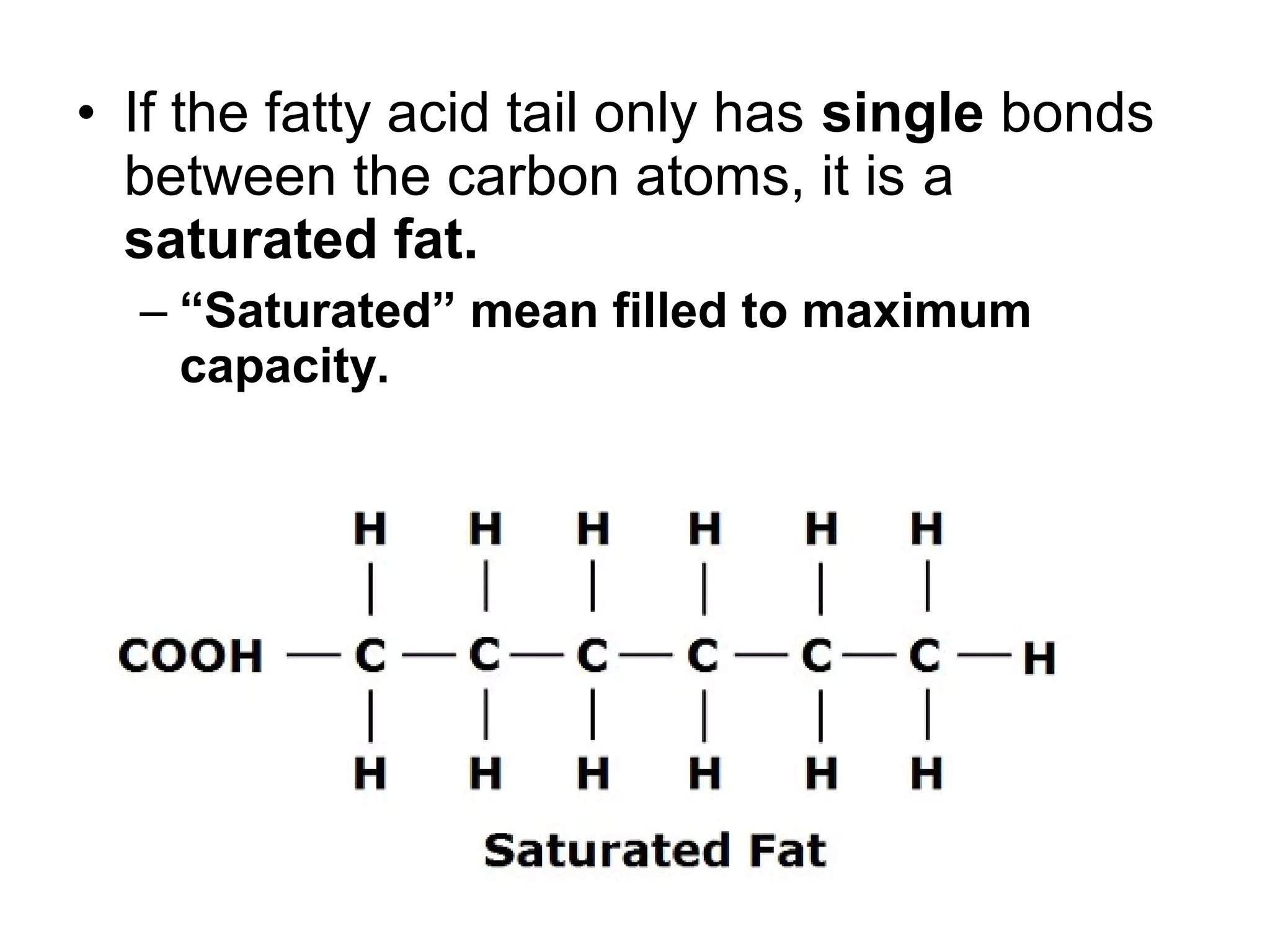
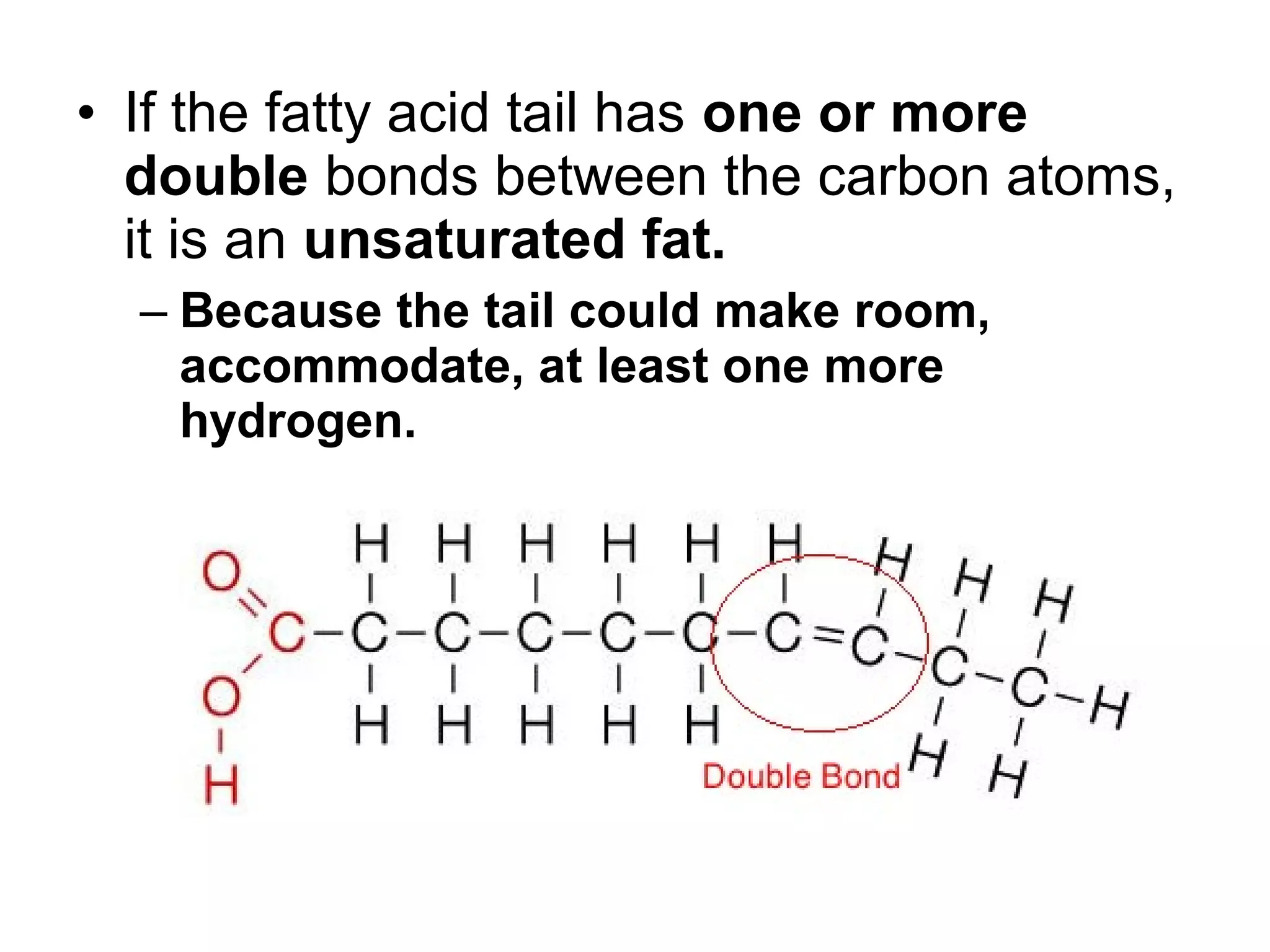
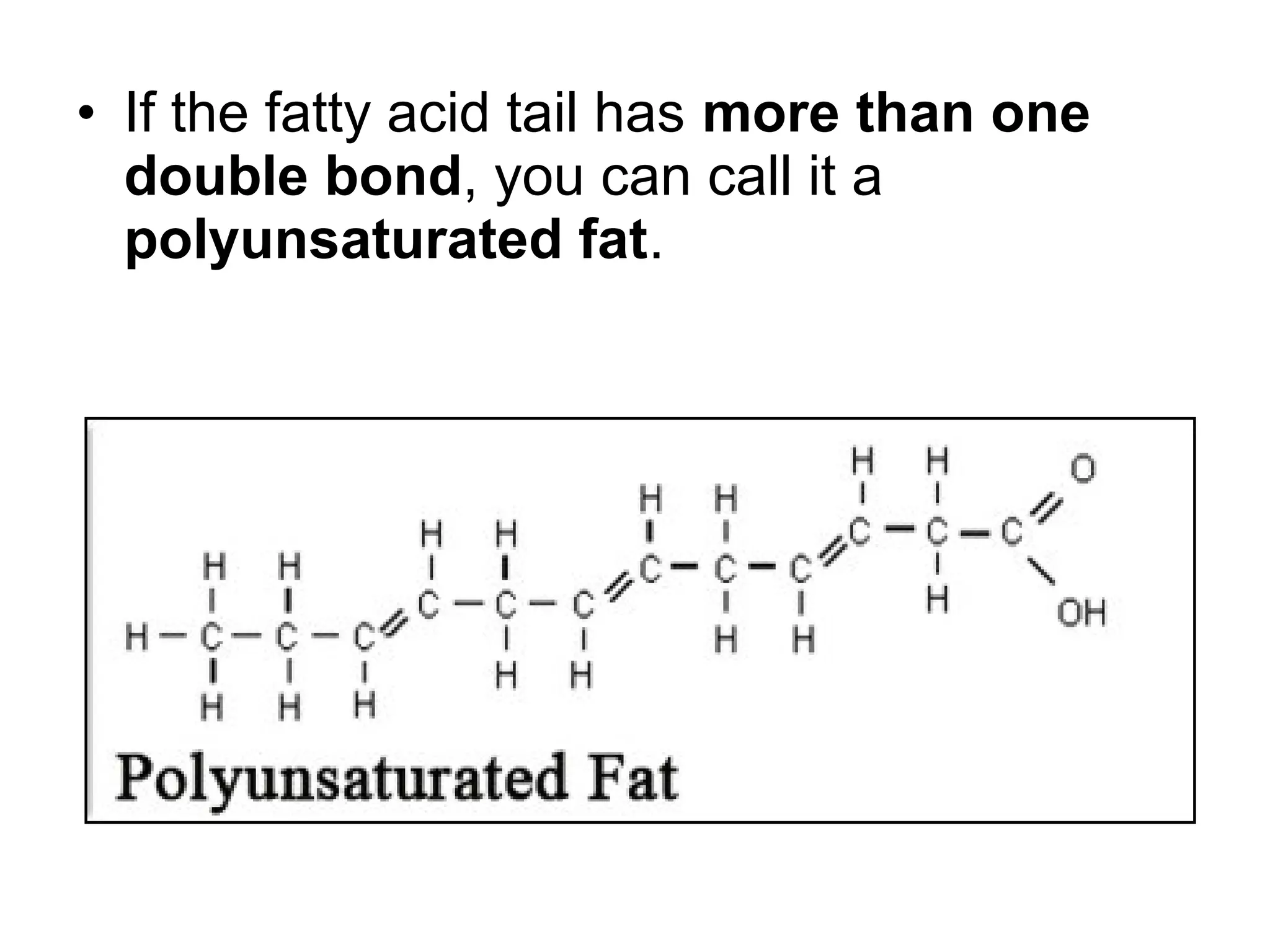
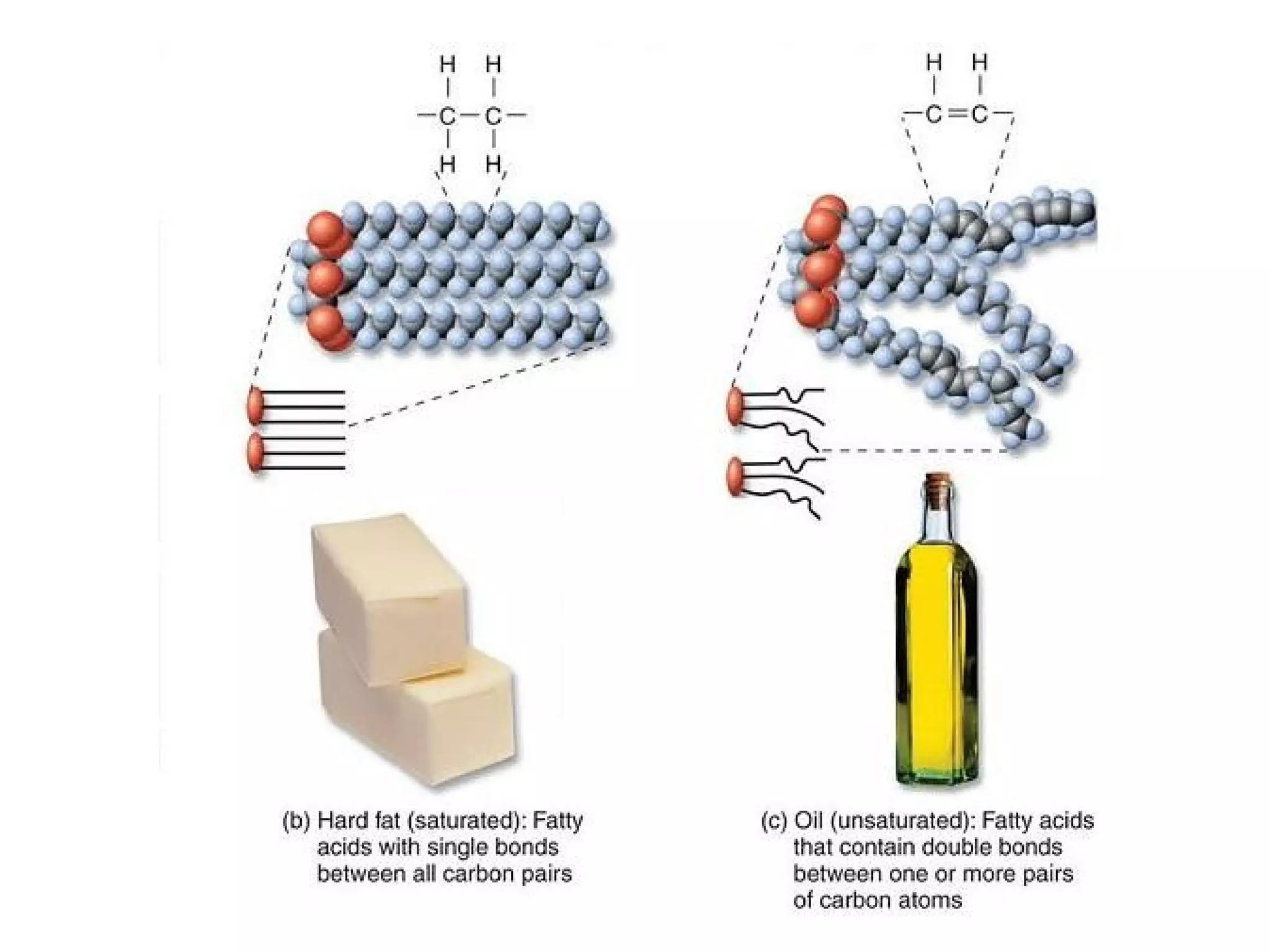
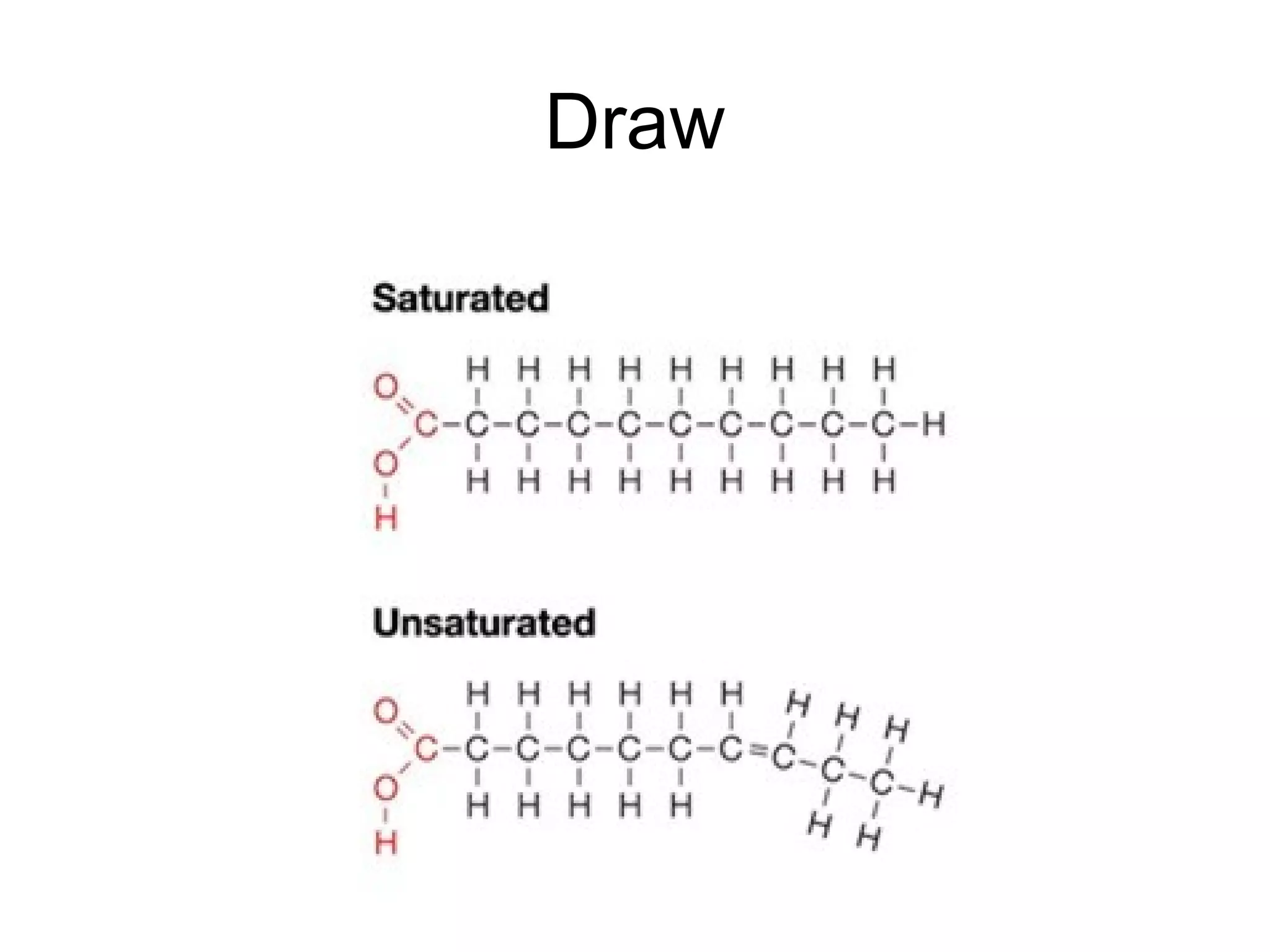
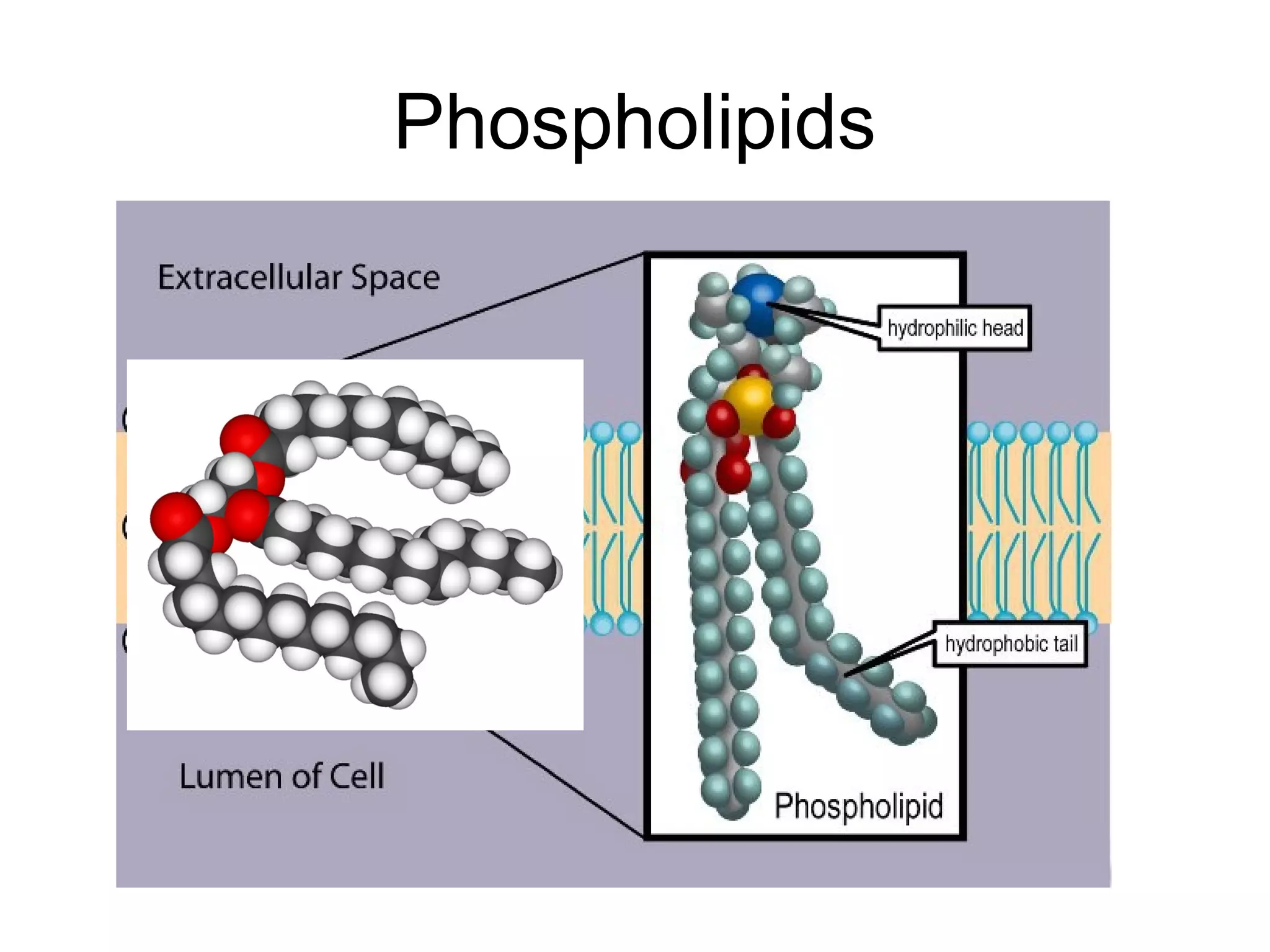
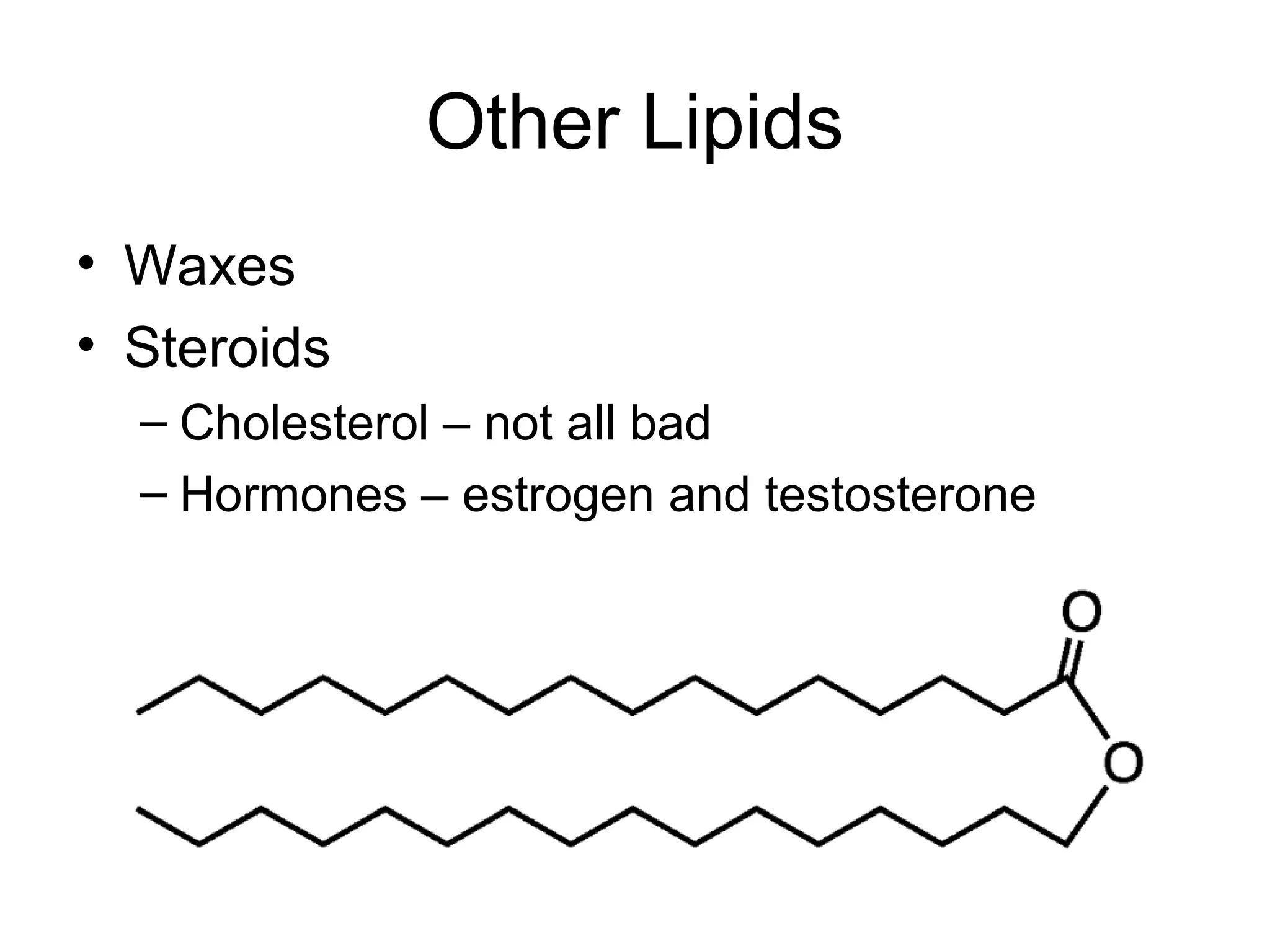
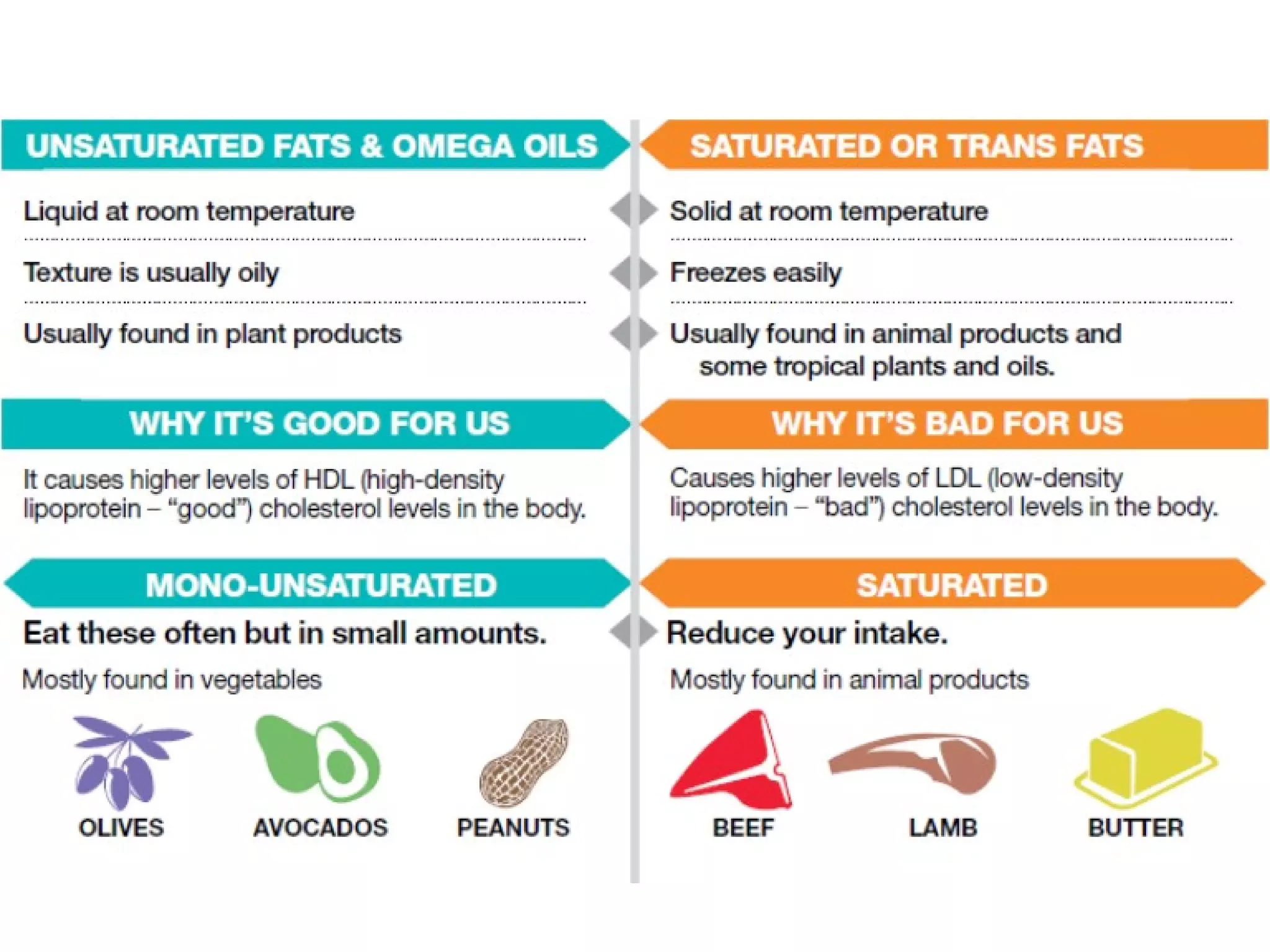
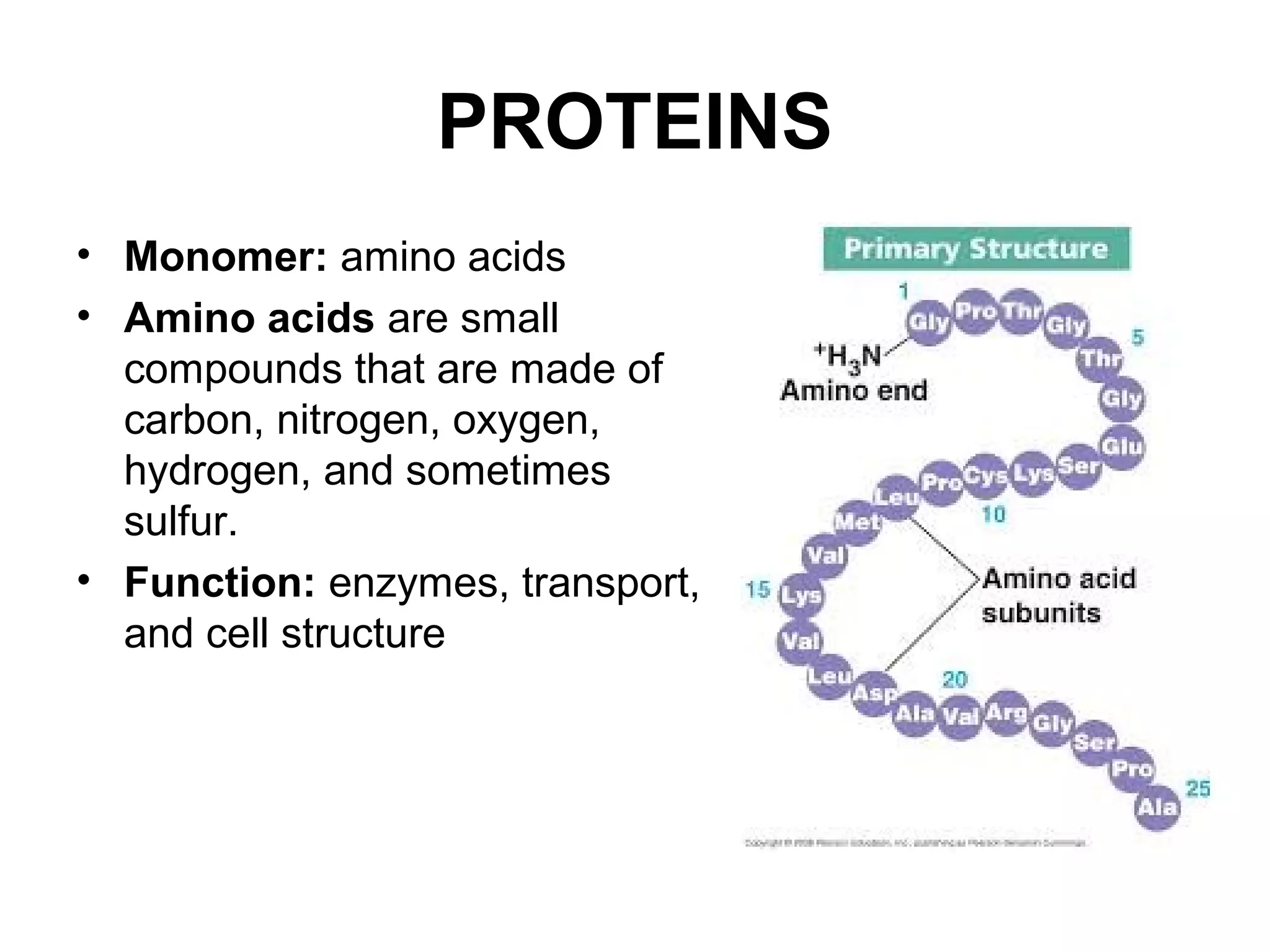
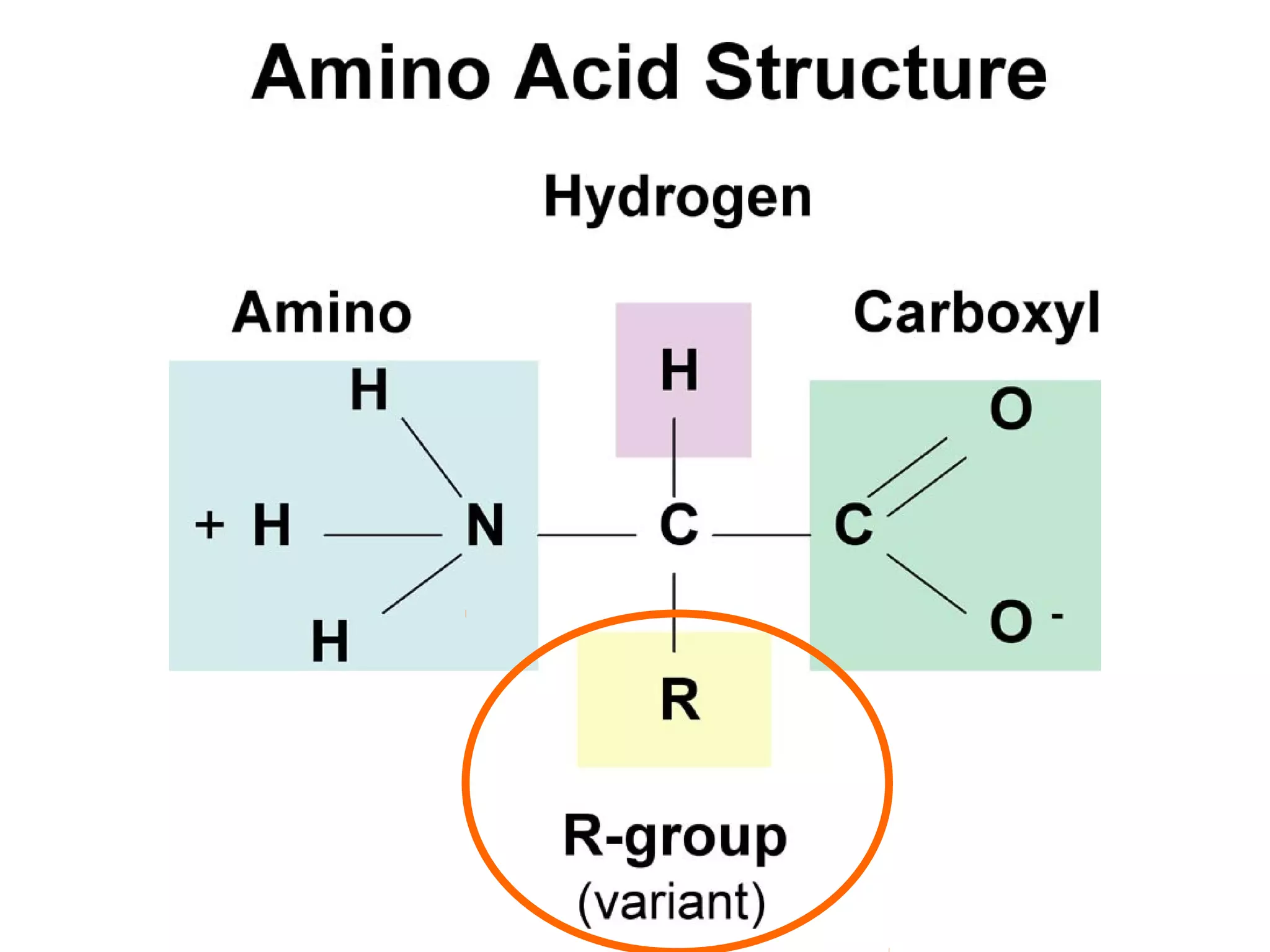
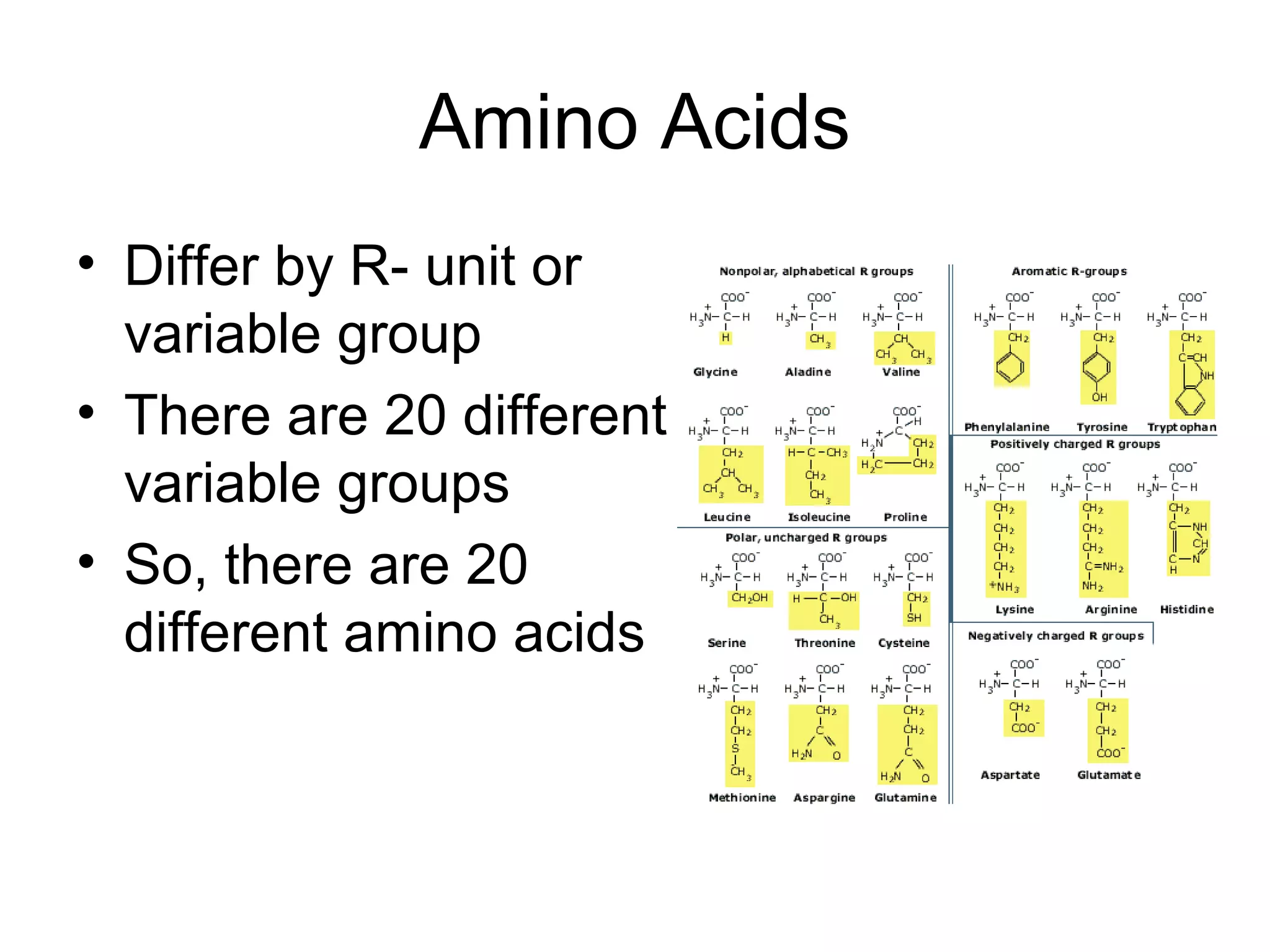
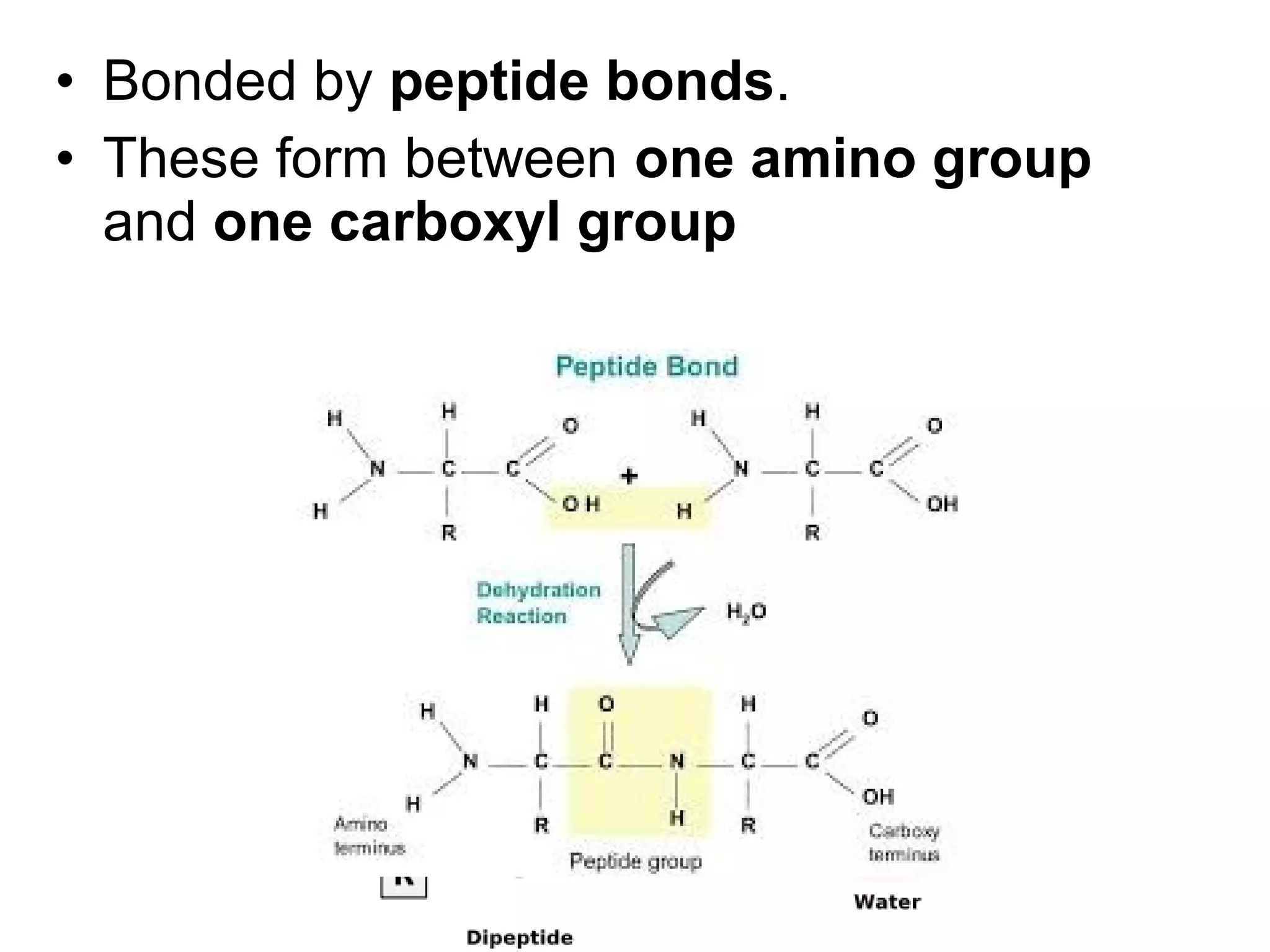
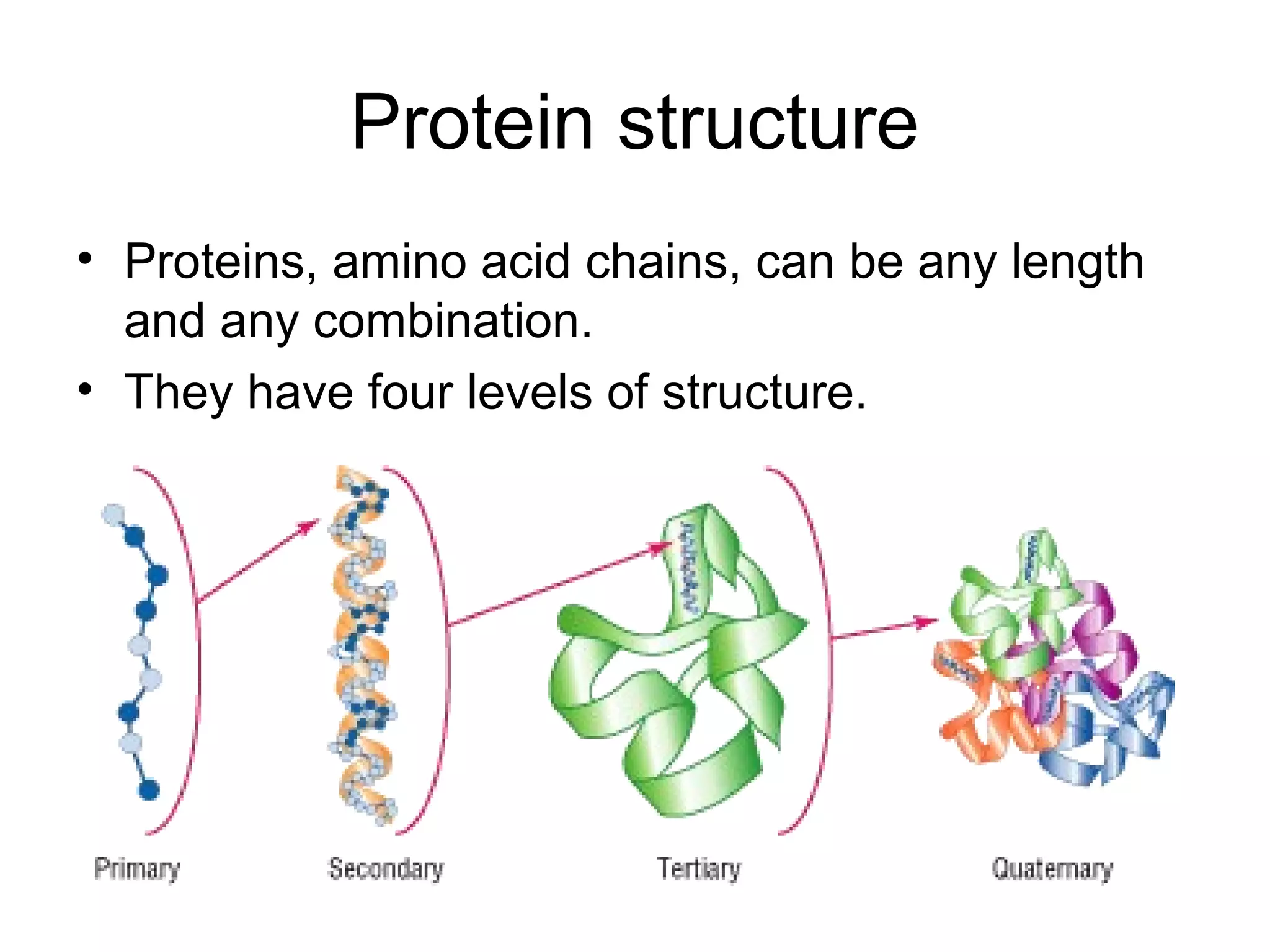
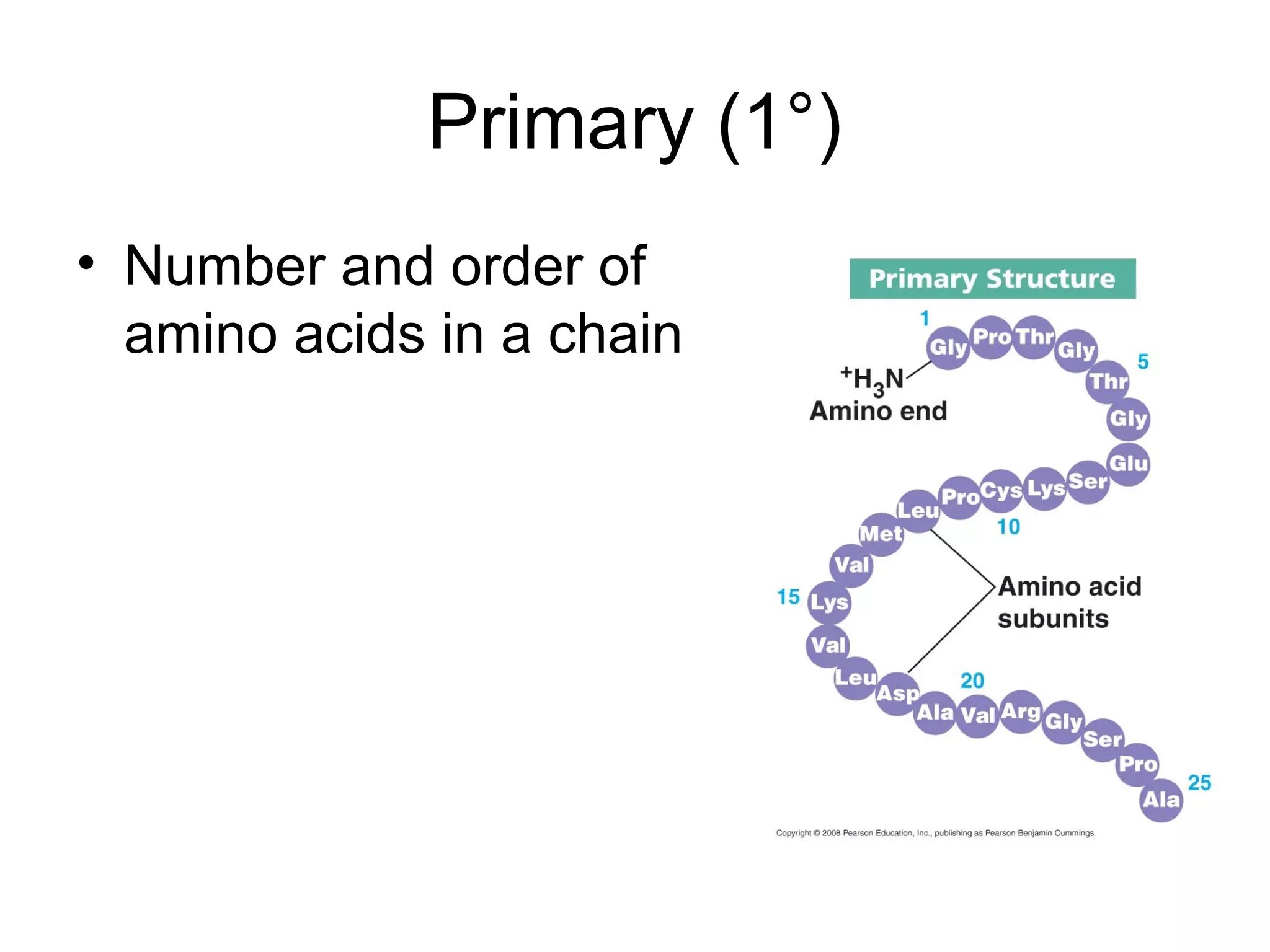
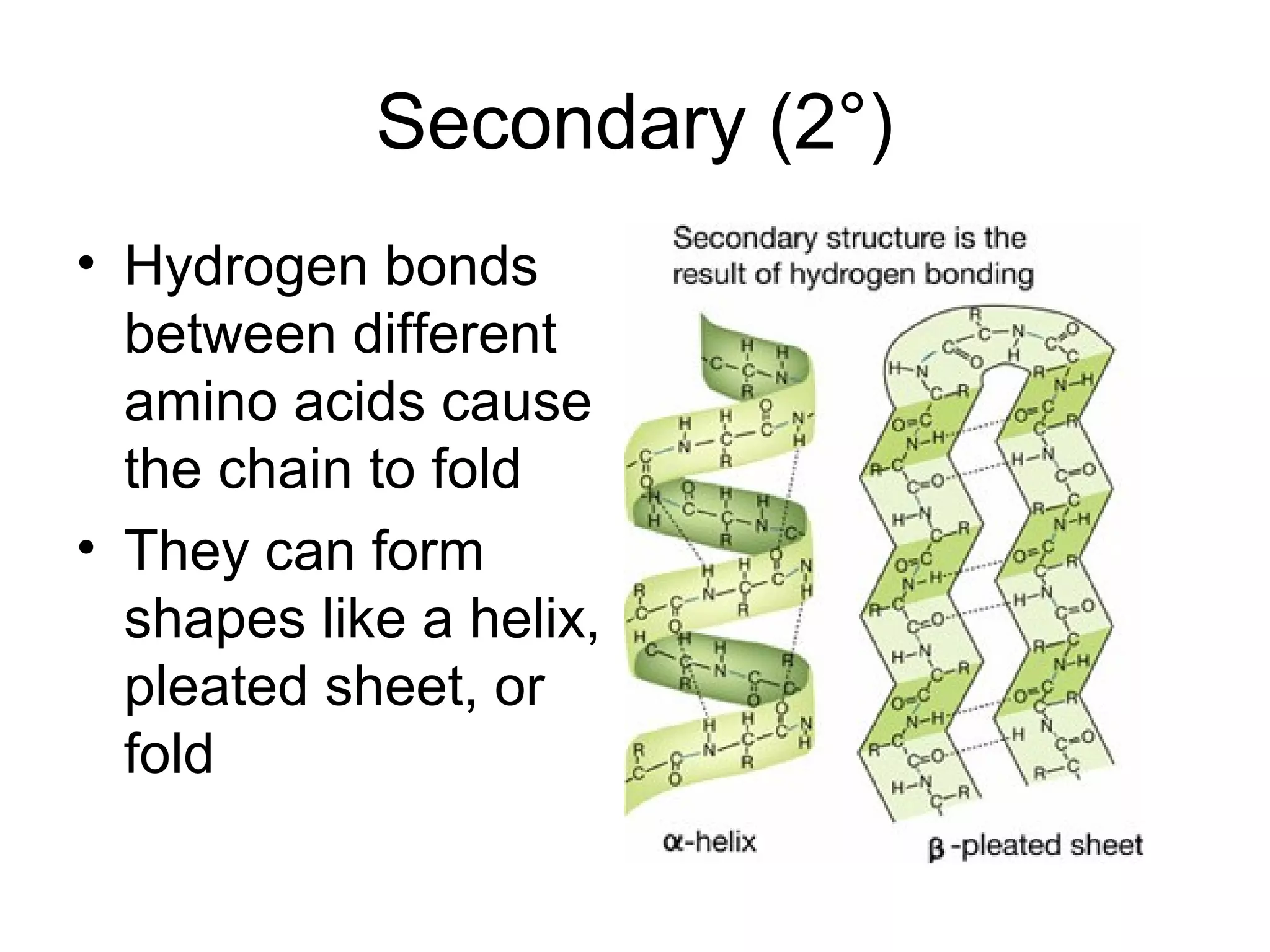
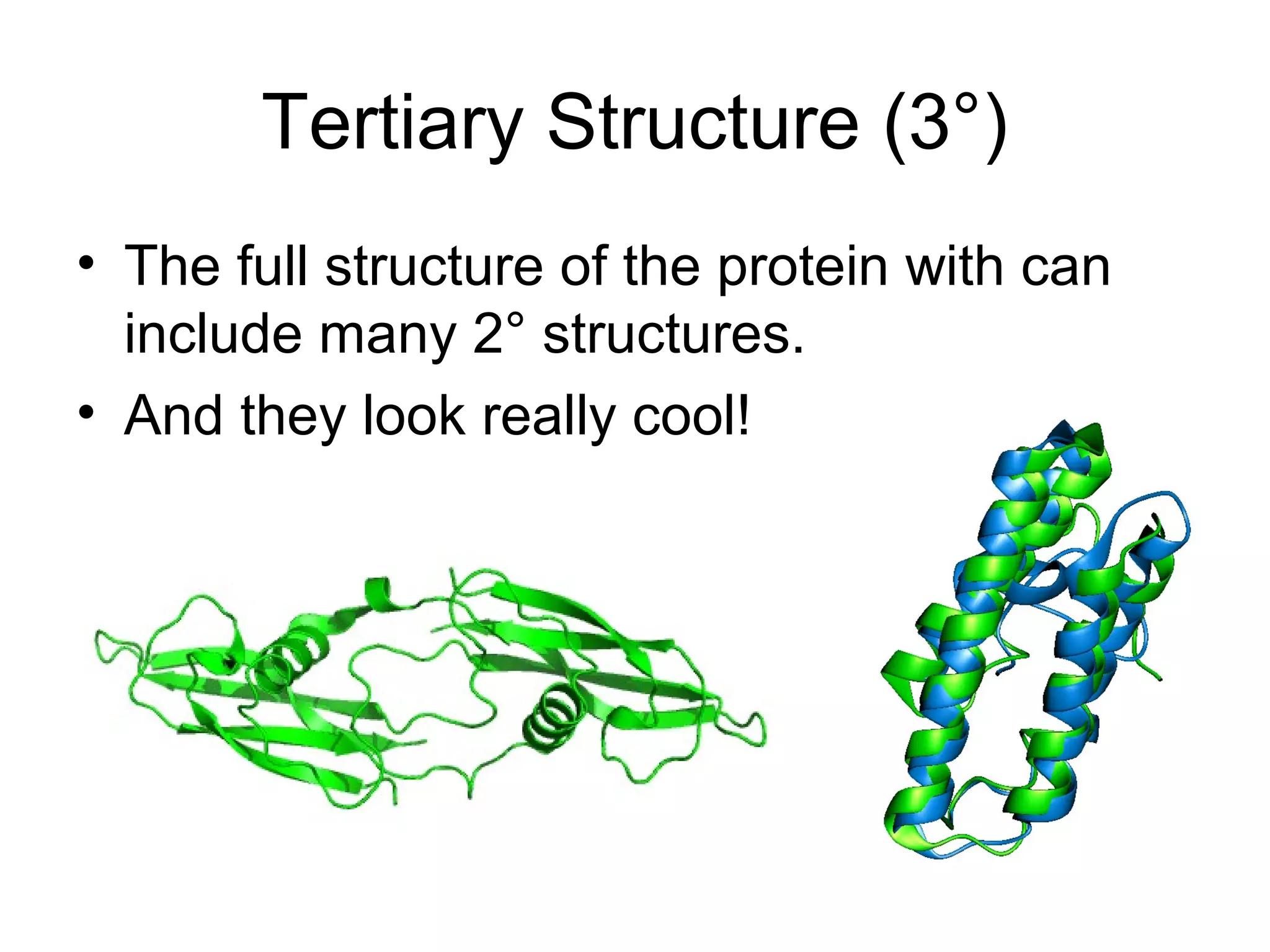
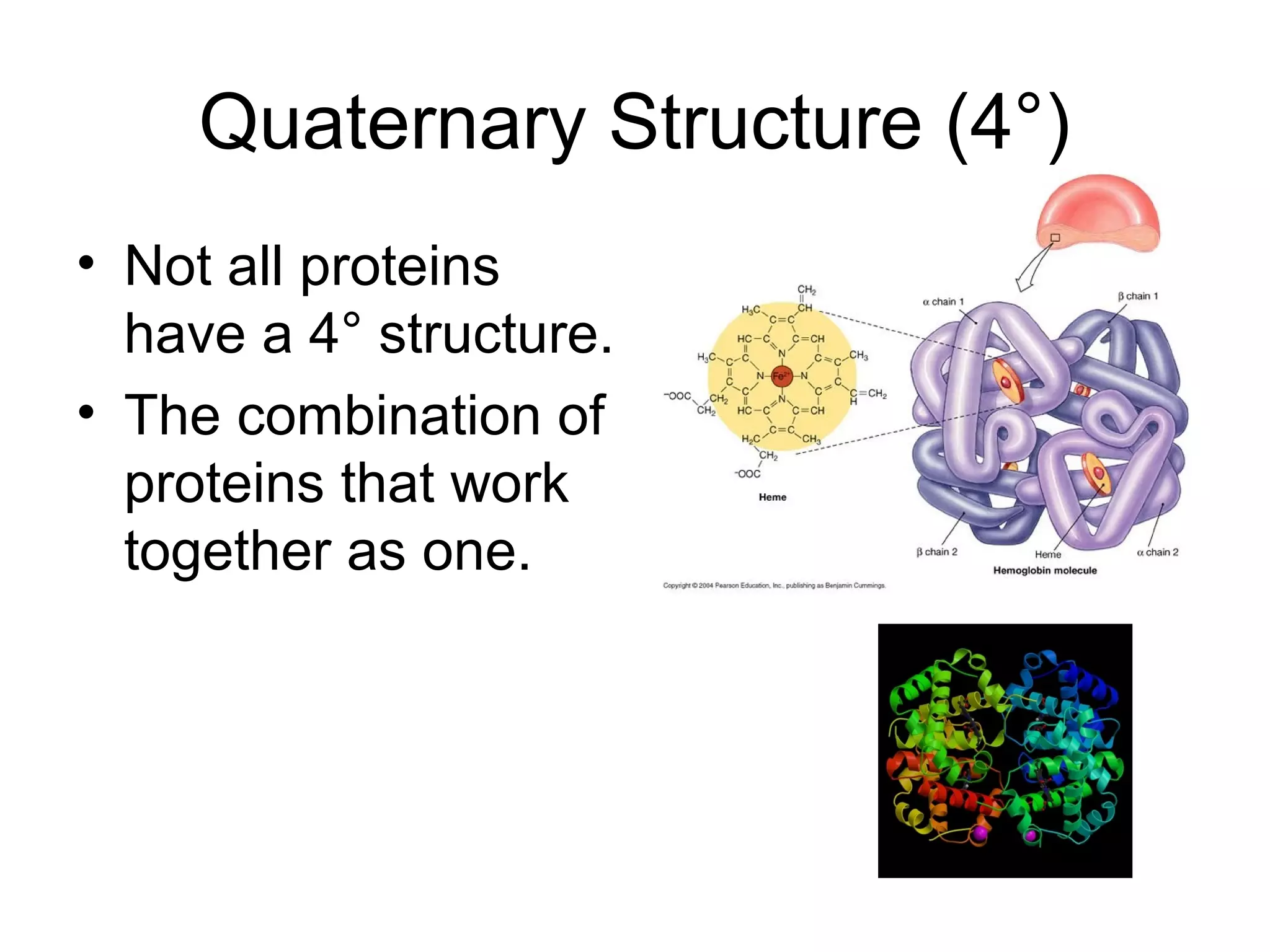
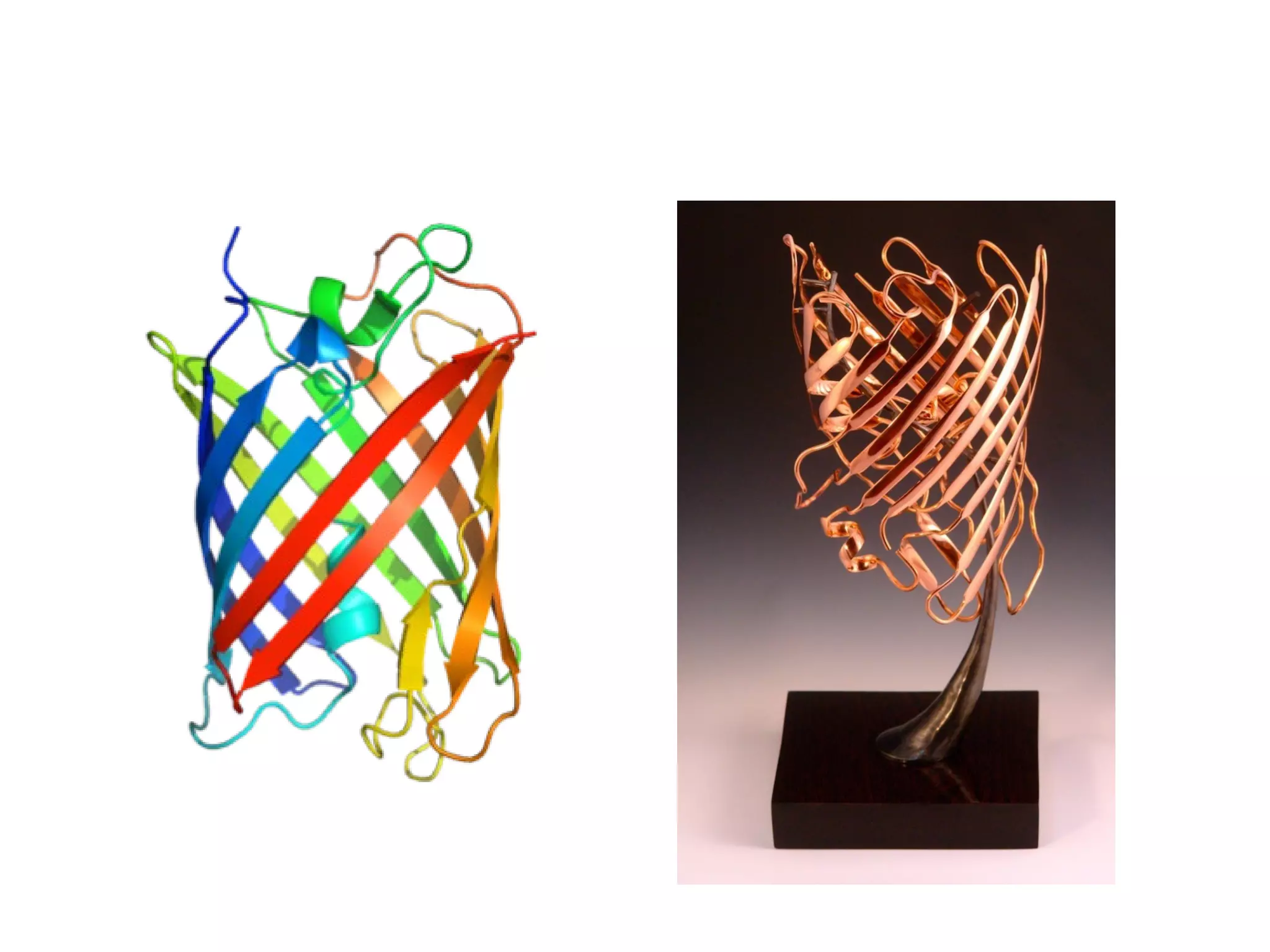
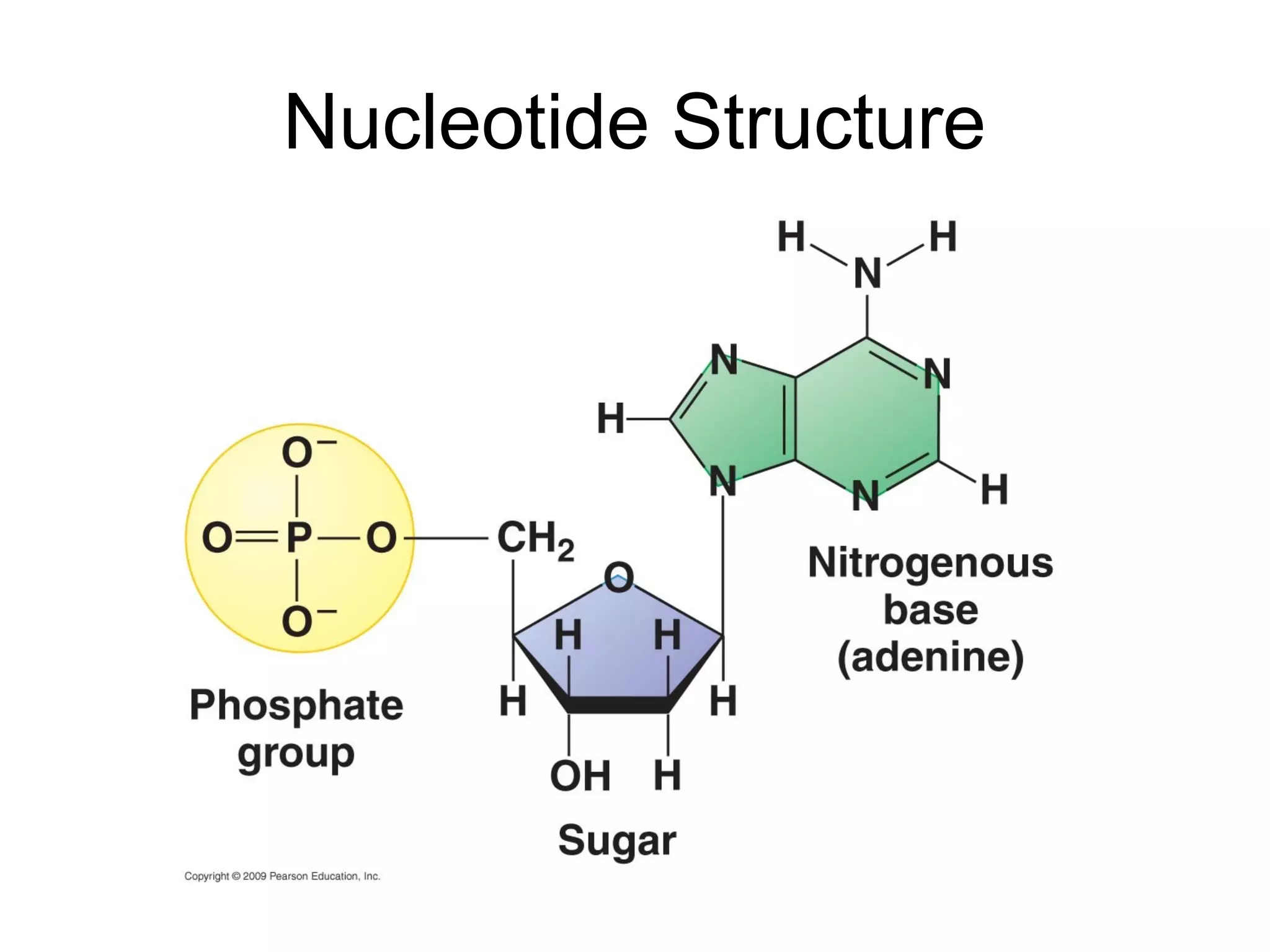
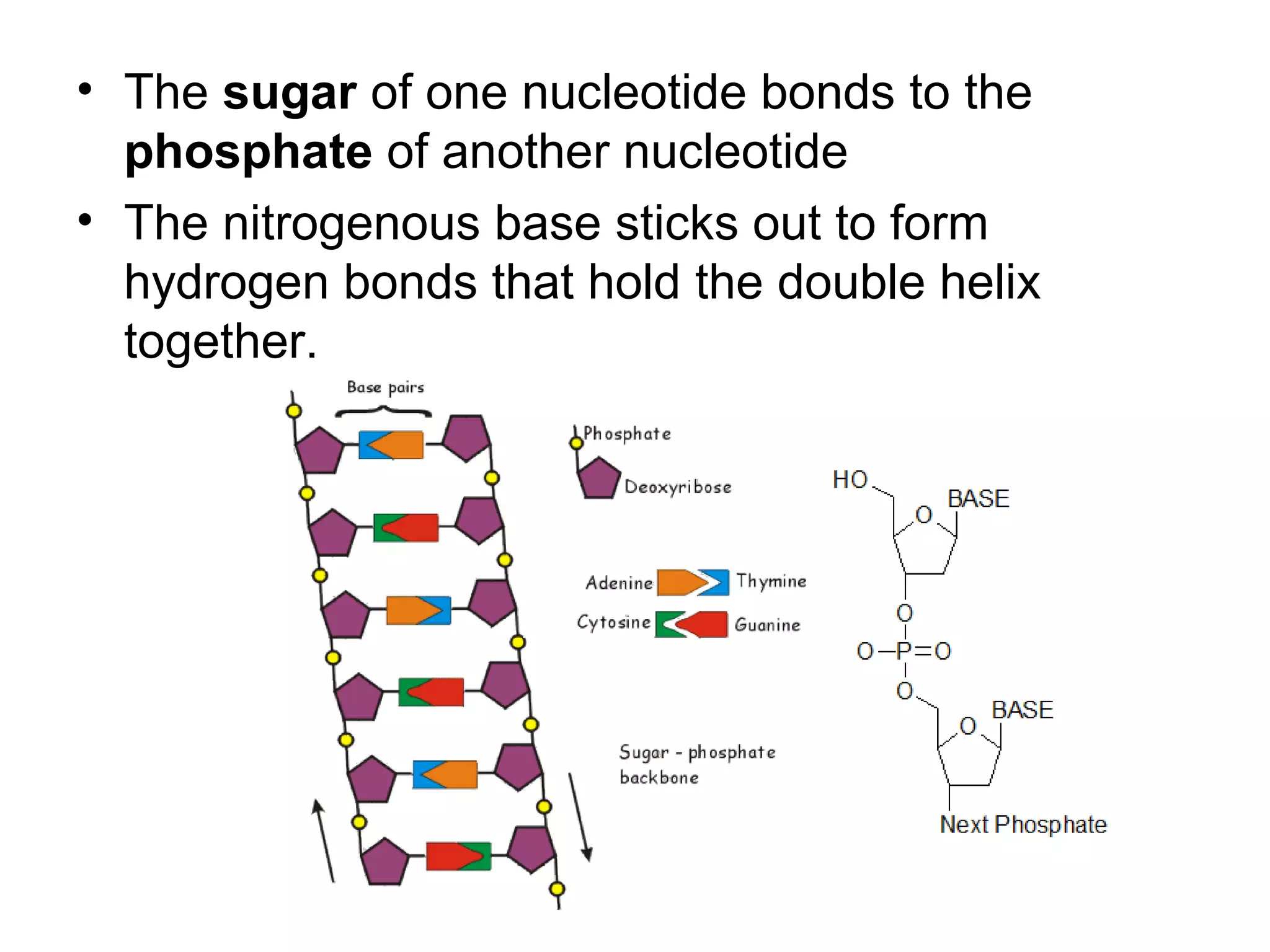
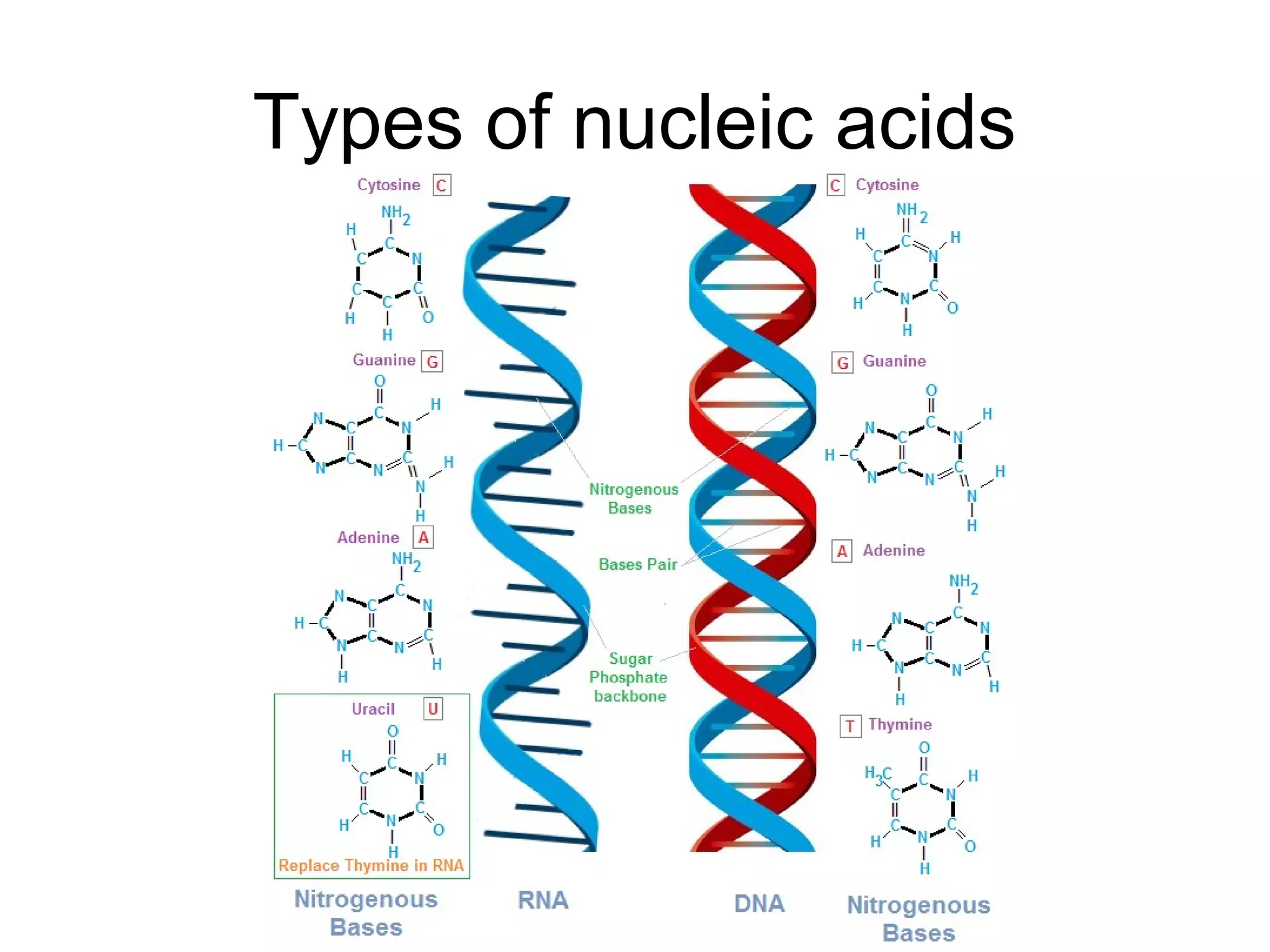
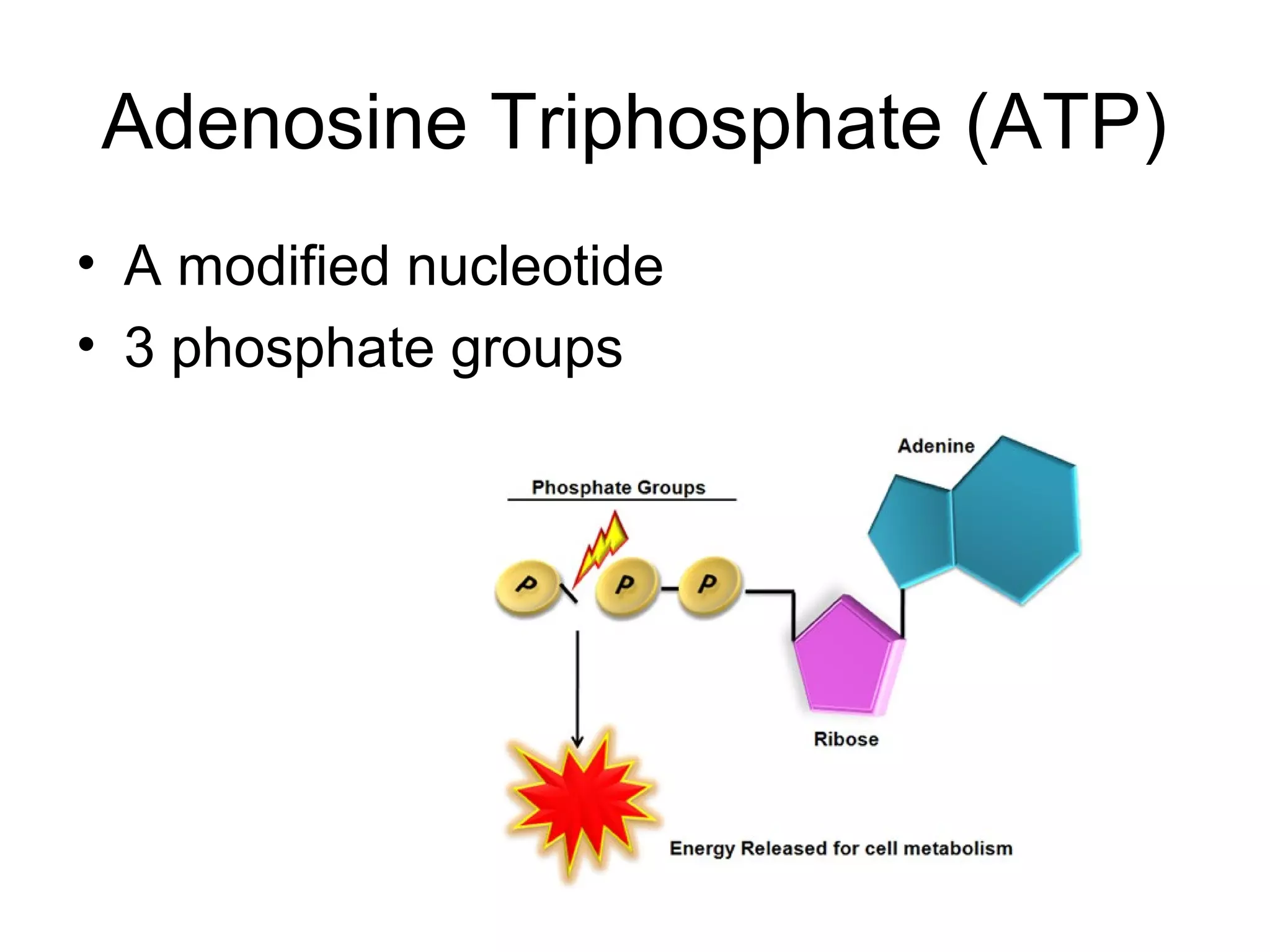
The document discusses the building blocks of life, focusing on macromolecules, which include carbohydrates, lipids, proteins, and nucleic acids, all essential for cellular structure and function. It explains the roles of these macromolecules, their monomers and polymers, and the significance of carbon in organic chemistry. Additionally, it highlights the structure and function of proteins and nucleic acids in storing and transmitting genetic information.