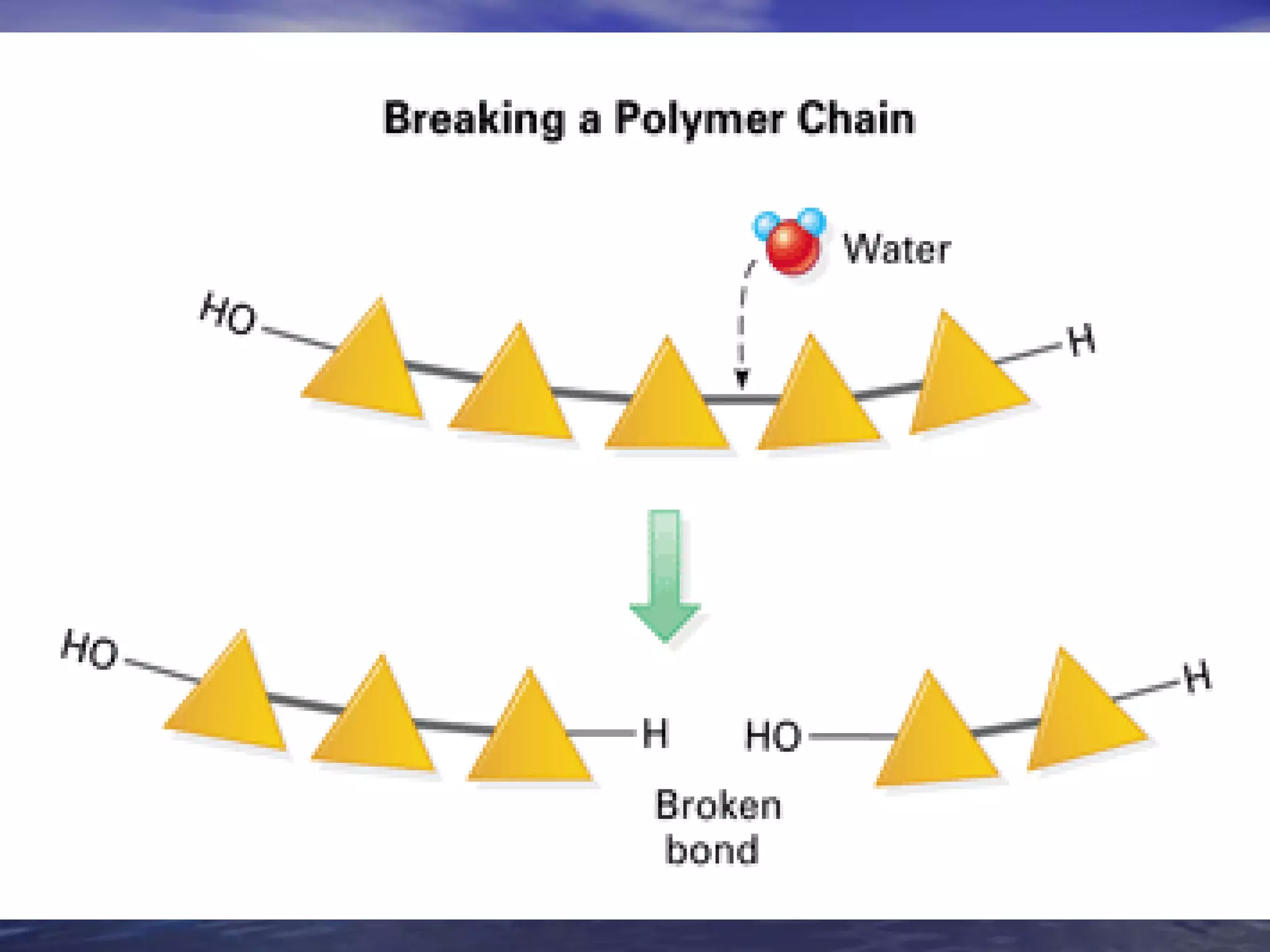
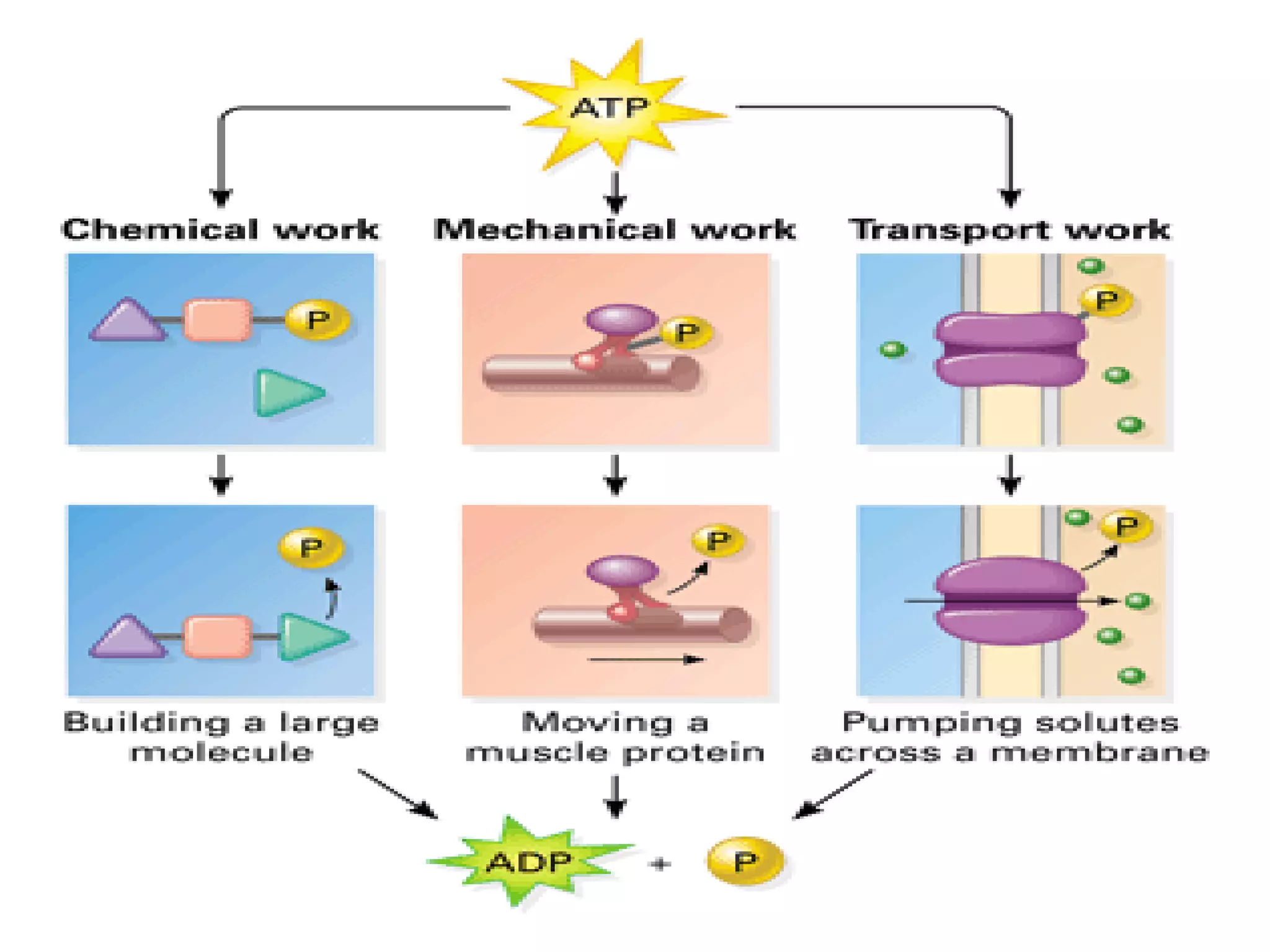
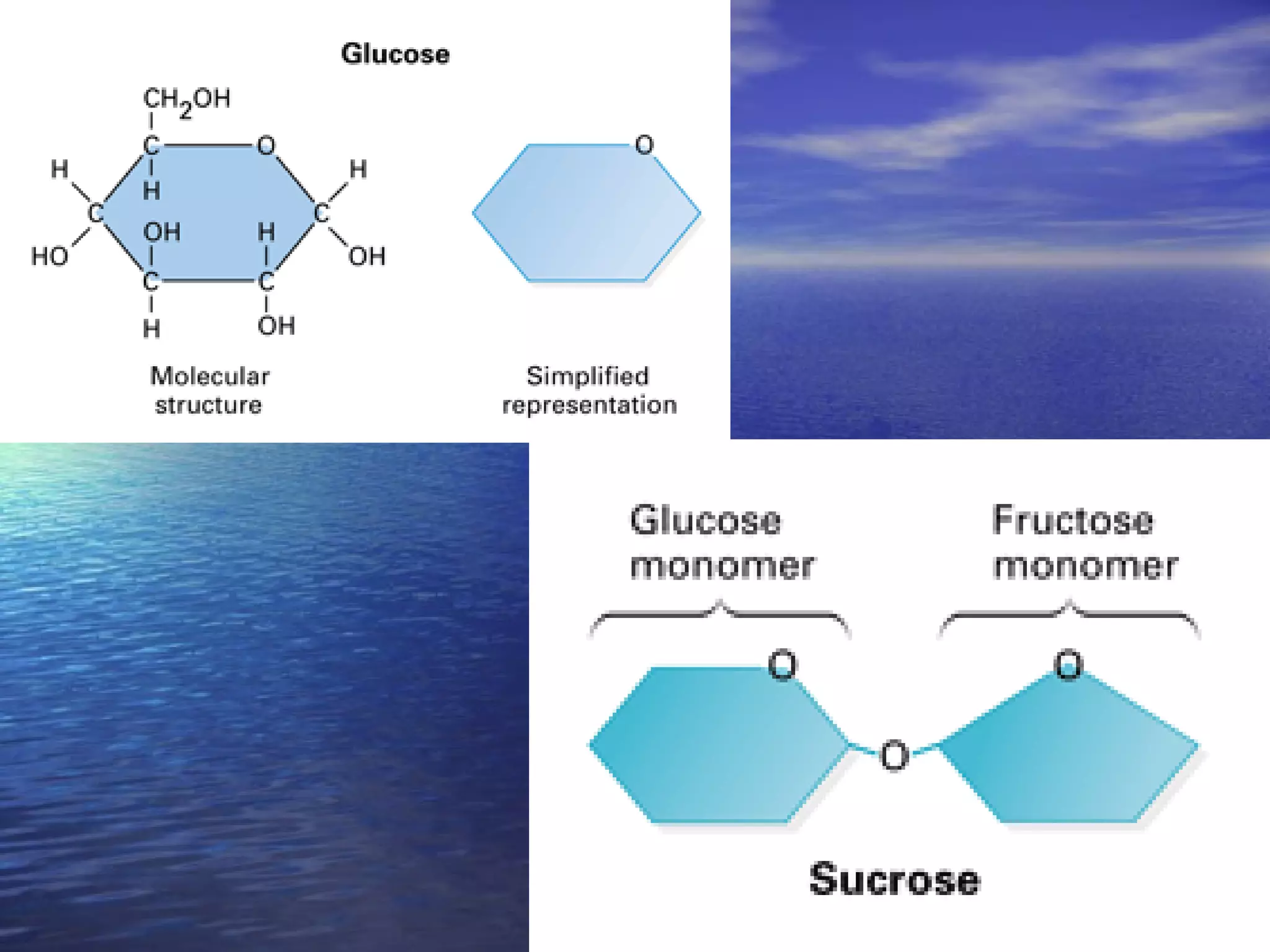
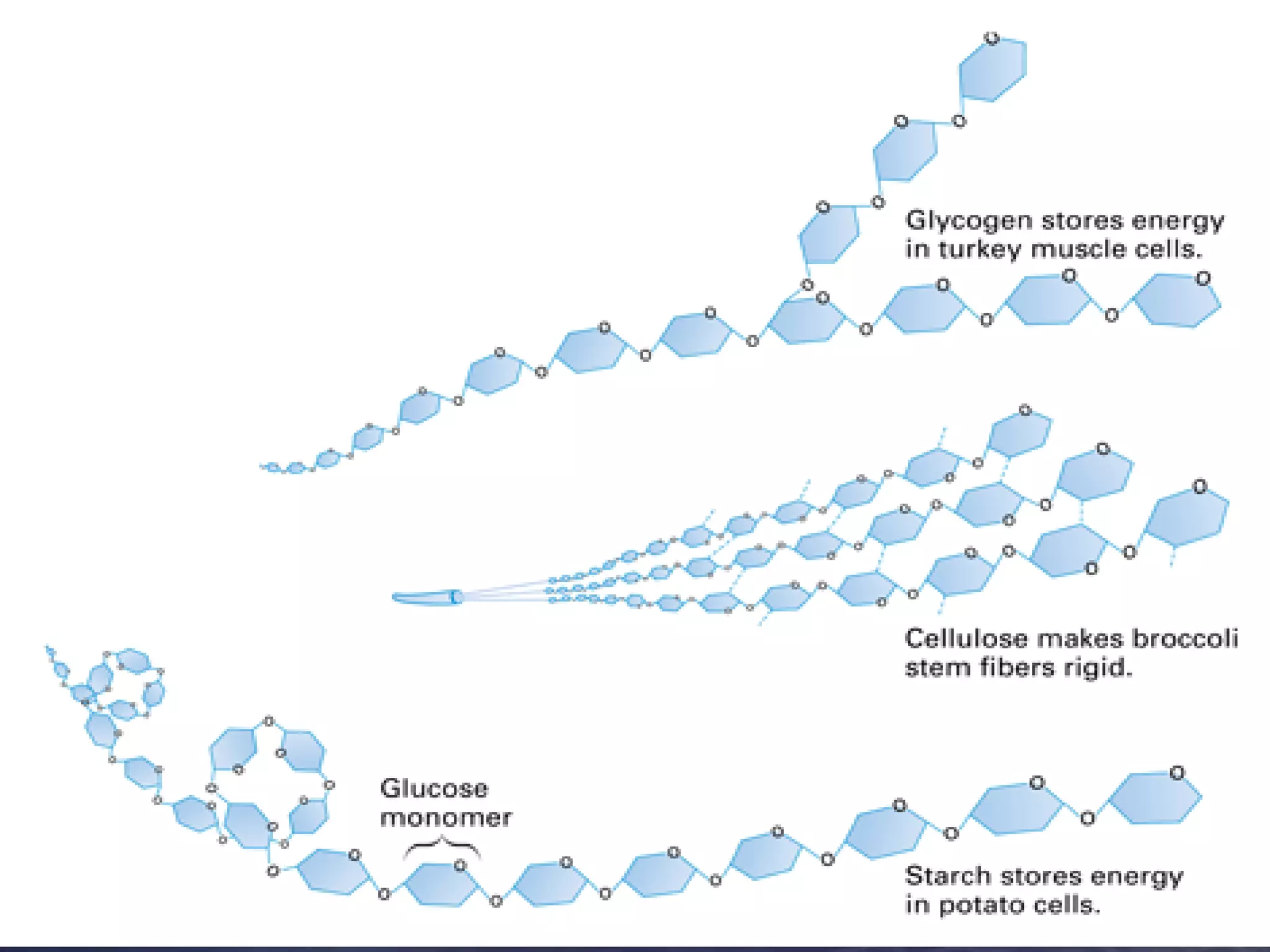
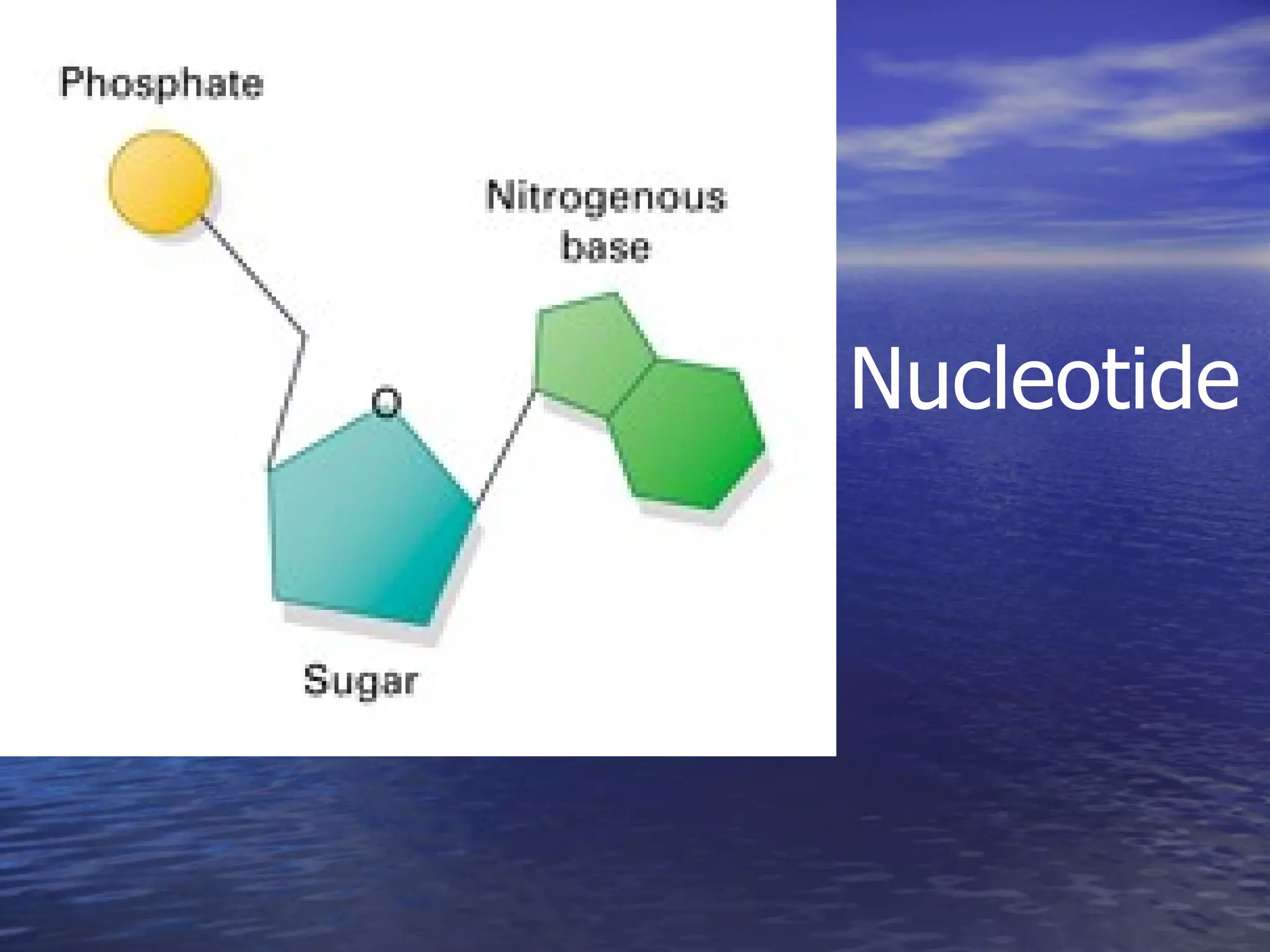
Water has 4 important properties: it is polar, can form hydrogen bonds, is cohesive, and is adhesive. Its polarity and ability to form hydrogen bonds allow it to have unique properties like surface tension and capillarity that are important for life. The 4 main classes of large organic molecules are carbohydrates, proteins, lipids, and nucleic acids. Carbohydrates, proteins, and nucleic acids are polymers formed from monomers joined by condensation reactions and broken down by hydrolysis. Enzymes are protein catalysts that facilitate reactions by lowering the activation energy; their function can be affected by factors like temperature, pH, and foreign chemicals.