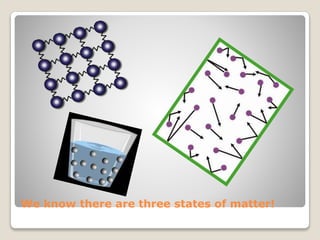
This document discusses the three states of matter - solids, liquids, and gases - and how changing temperature can cause matter to change states. It uses water as an example, explaining that heating ice causes it to melt into liquid water, heating the liquid water causes it to boil and become steam gas, and cooling steam causes it to condense back into liquid water. The key point is that raising or lowering the temperature of matter can change its state from solid to liquid to gas or back again.