The document discusses different types of phase changes:
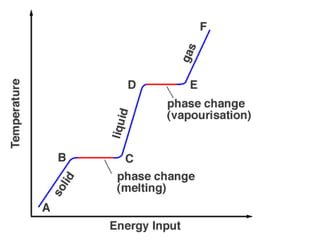
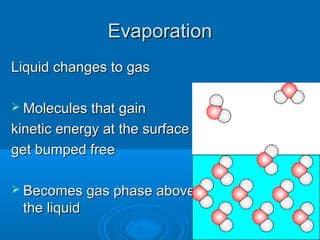
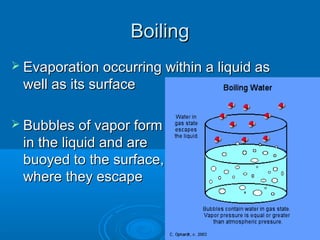
1) Evaporation is when liquid changes to gas as molecules gain kinetic energy and leave the surface. Boiling is evaporation occurring within the liquid as bubbles form.
2) Condensation is the opposite, when gas changes to liquid as high energy gas molecules hit the liquid surface.
3) Melting is when a solid changes to a liquid as heat is absorbed and molecules vibrate more. Freezing is the reverse as energy is removed and molecules slow down.
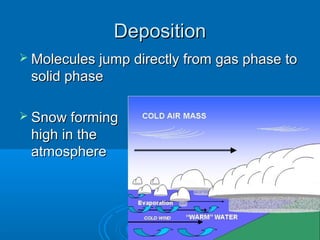
4) Sublimation and deposition involve direct changes between solid and gas phases.