Embed presentation
Download to read offline


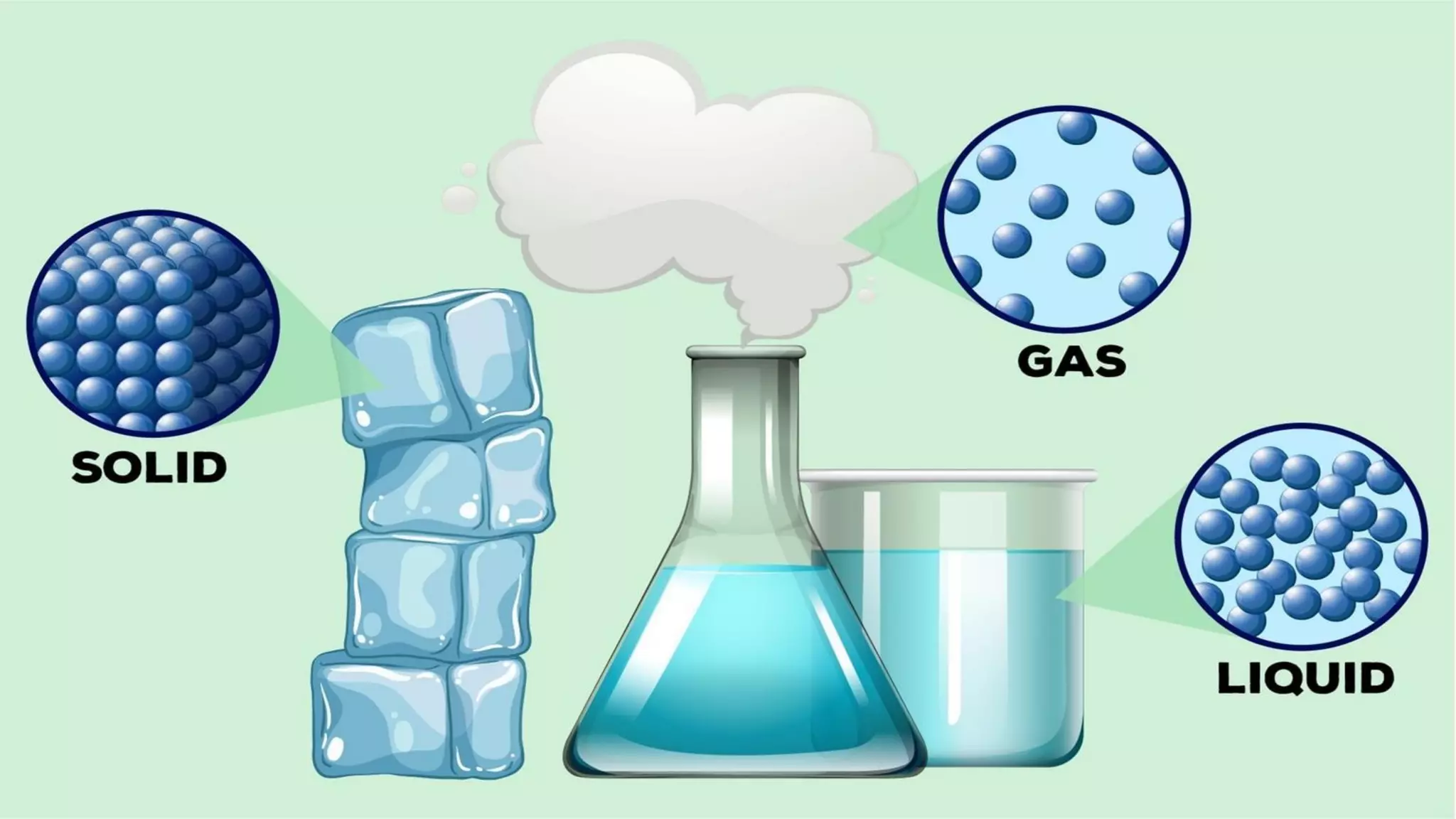








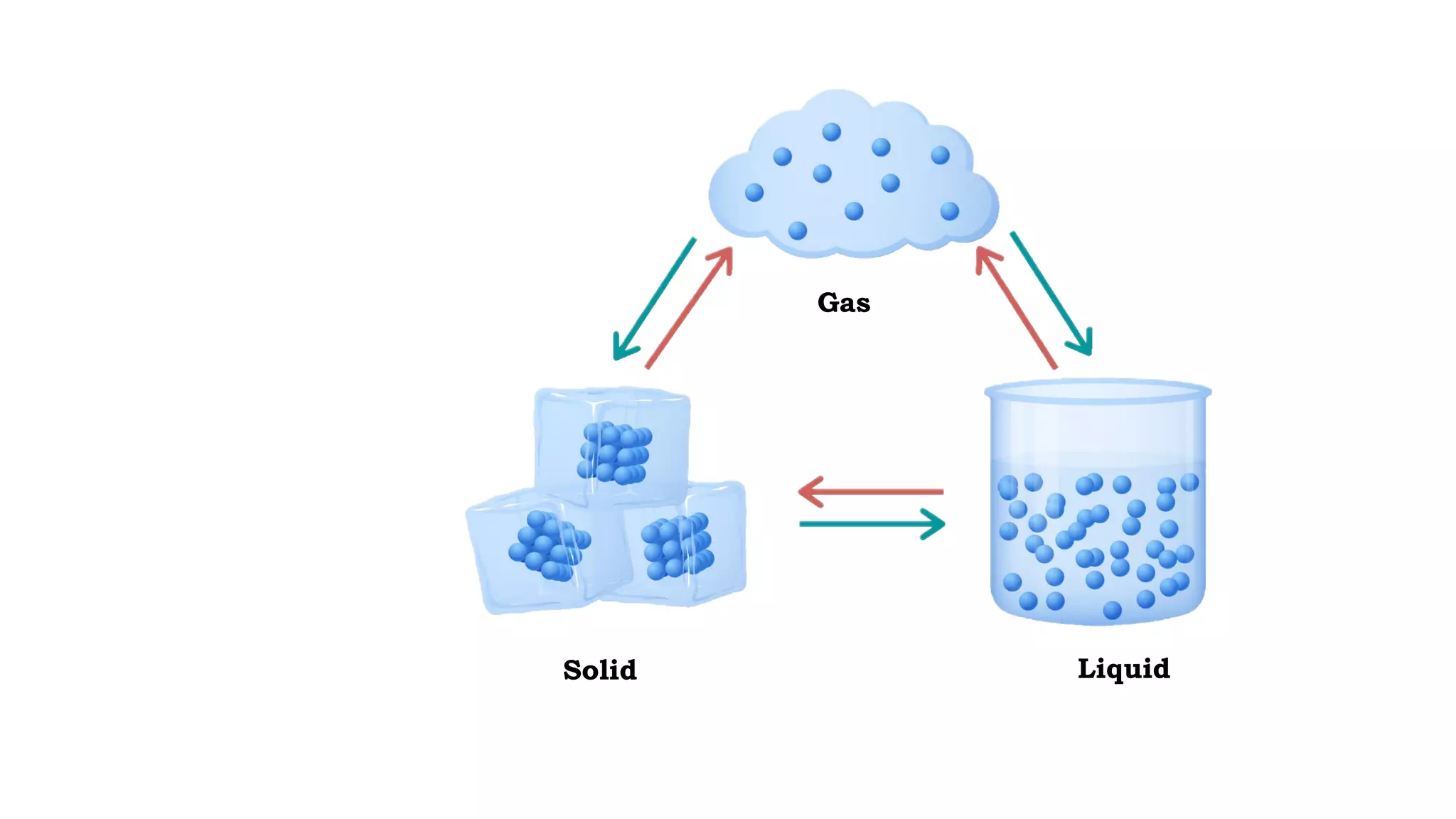





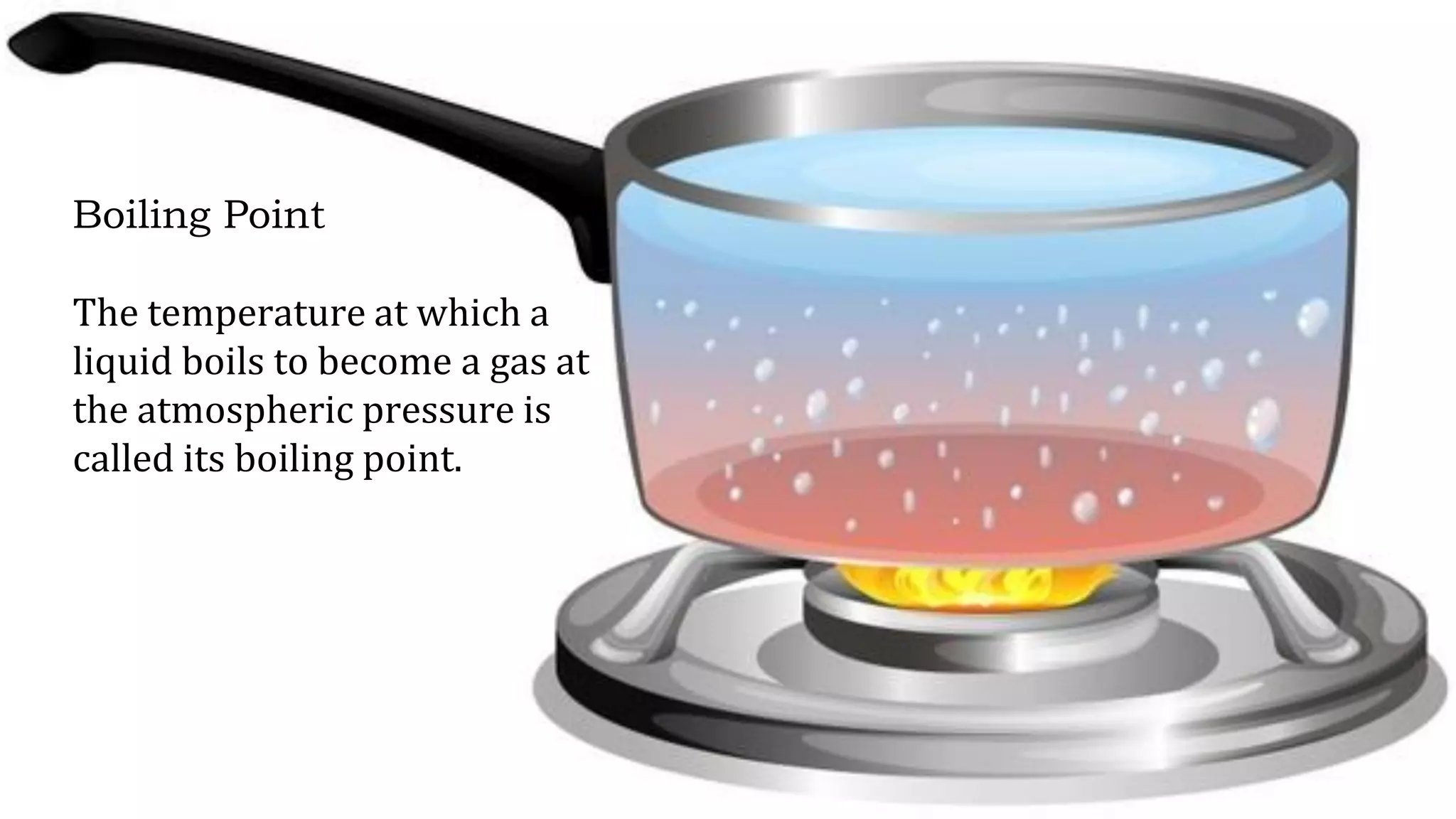
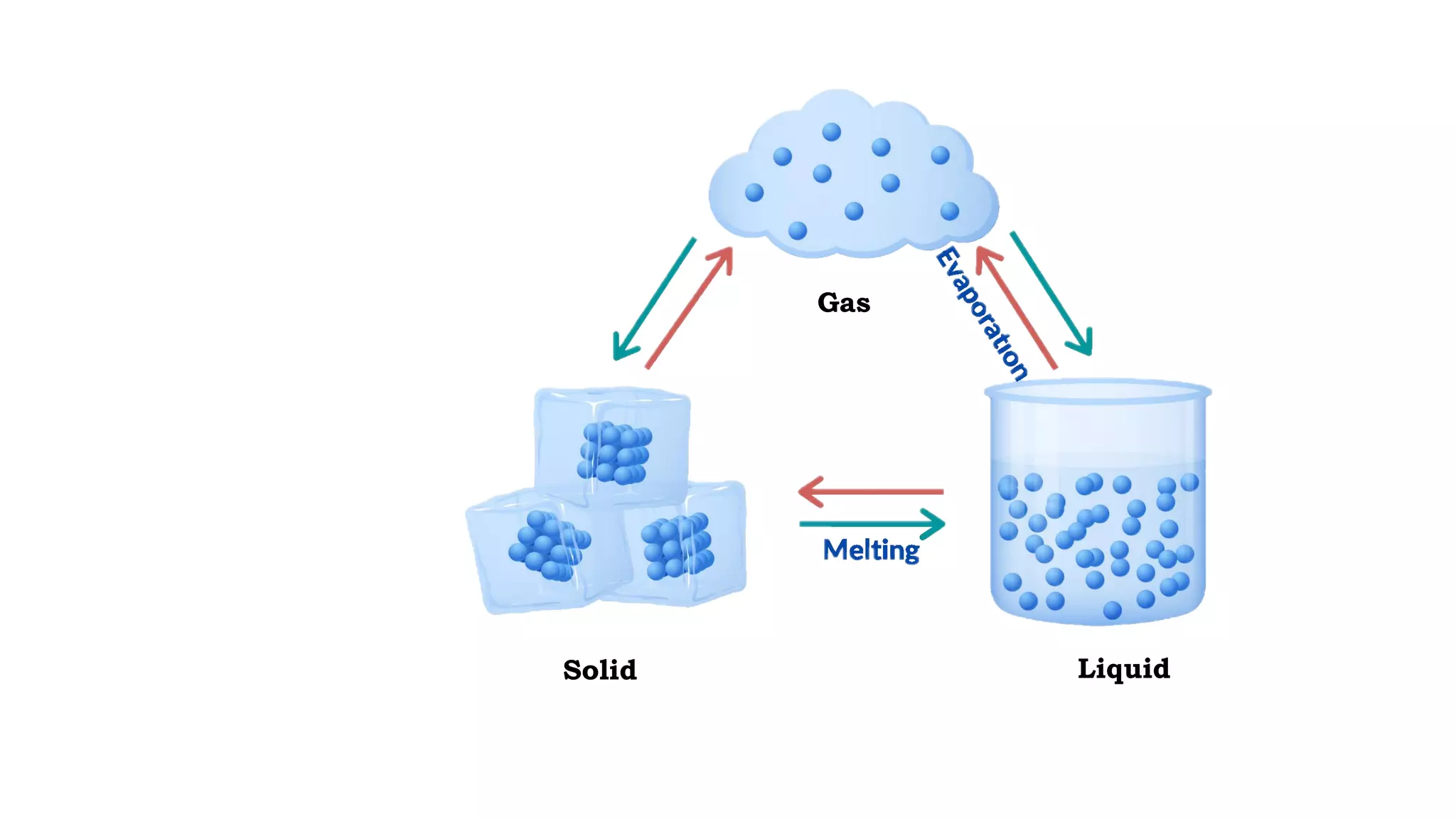


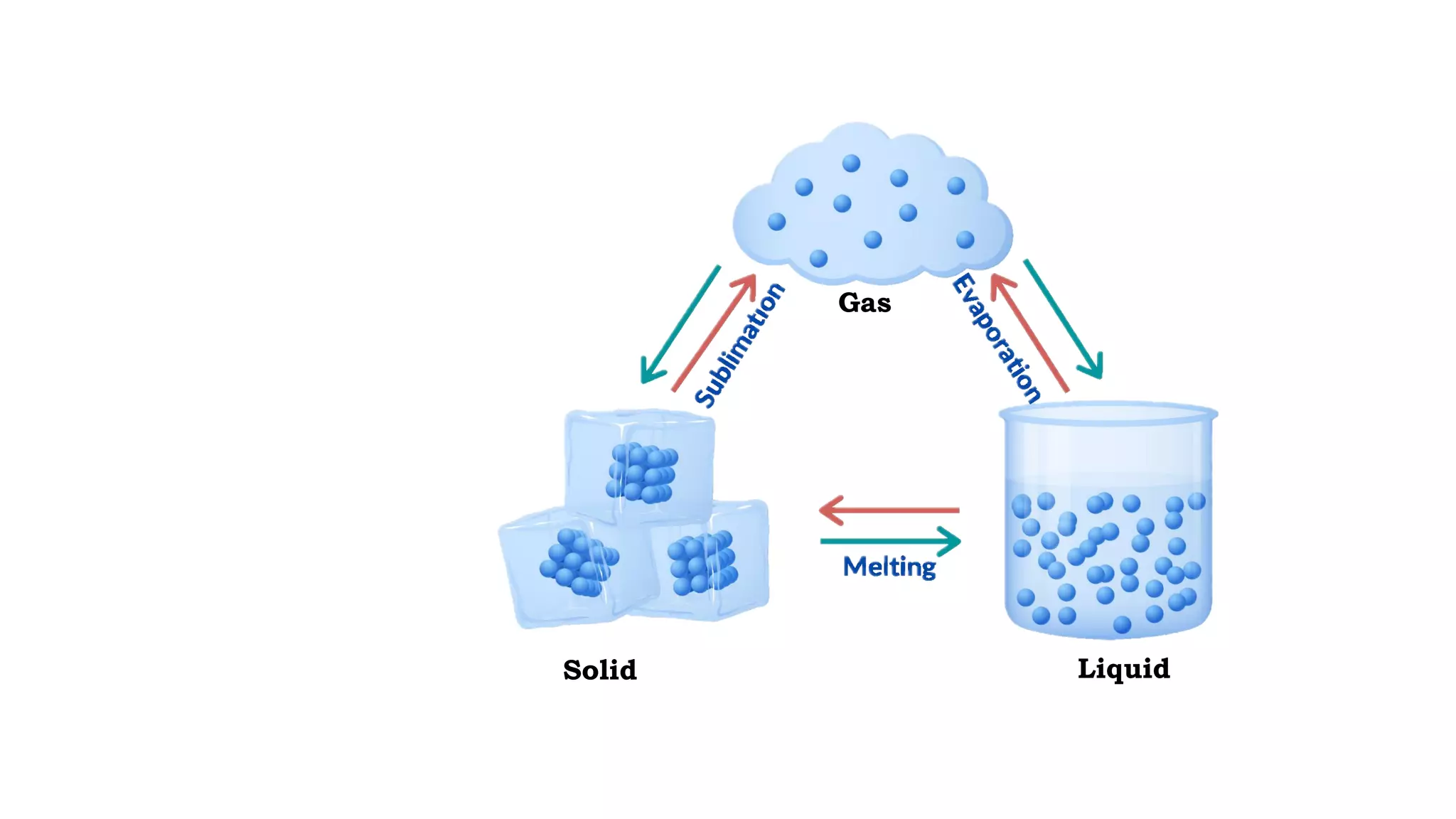



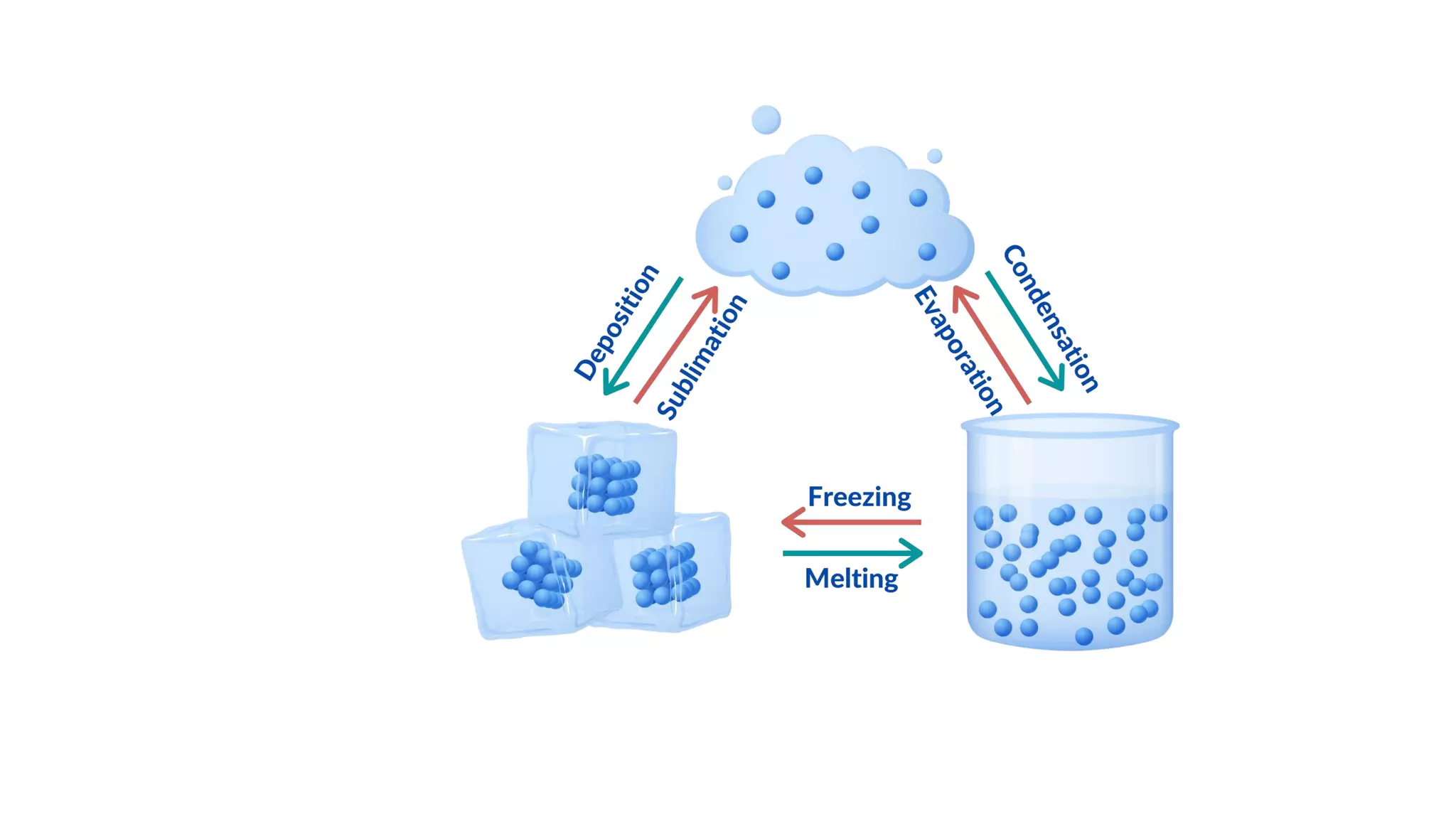


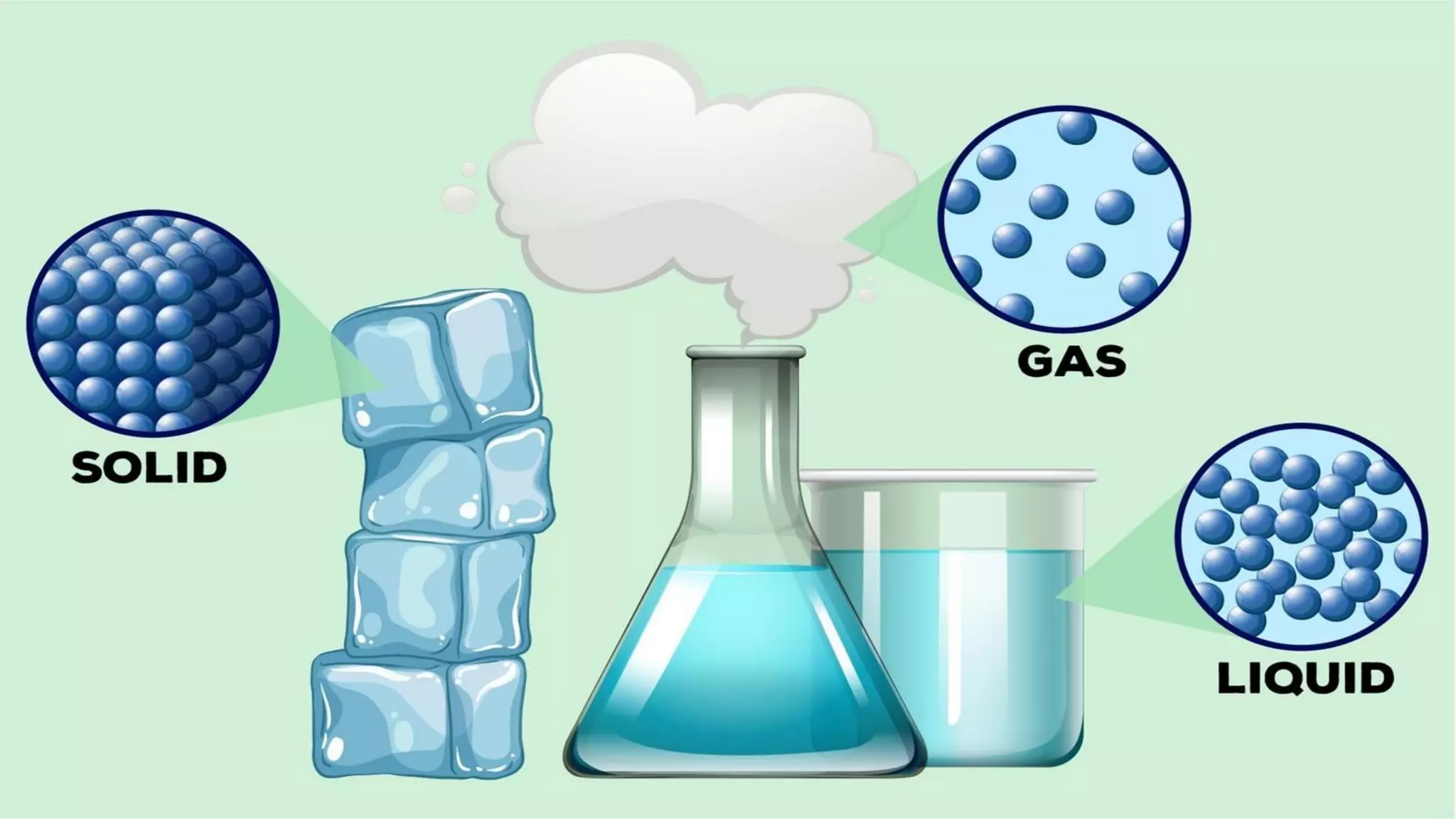


The document discusses different physical changes that occur when matter changes states between solid, liquid, and gas. It defines melting as when a solid turns to a liquid, boiling as when a liquid turns to a gas, and freezing and condensation as the reverse processes. The melting point of ice is given as 0°C, and boiling point is defined as the temperature at which a liquid boils and becomes a gas at atmospheric pressure. Sublimation and deposition are also introduced as changes directly between solid and gas states.































