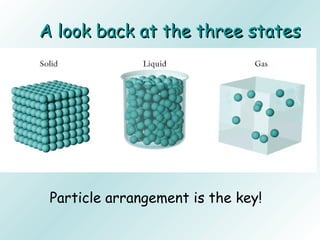
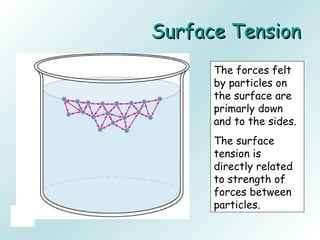
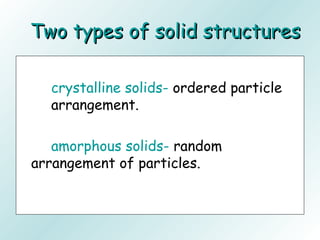
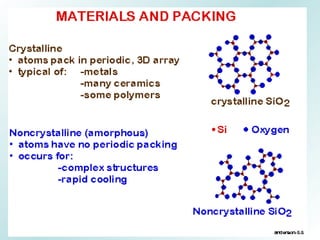
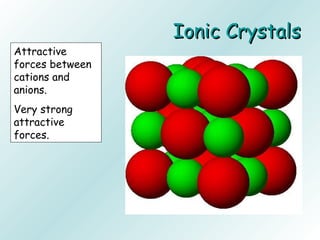
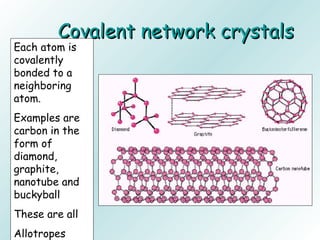
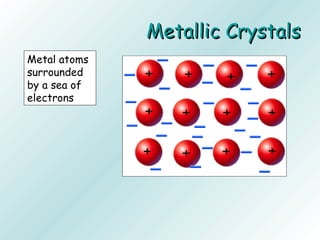
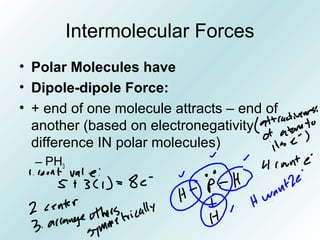
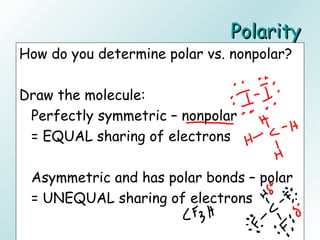
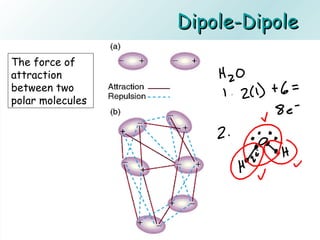
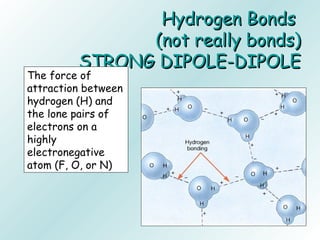
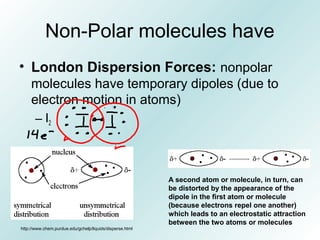
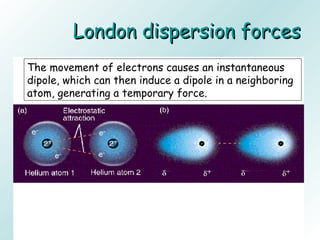
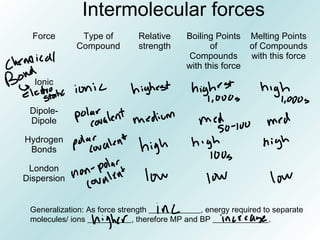
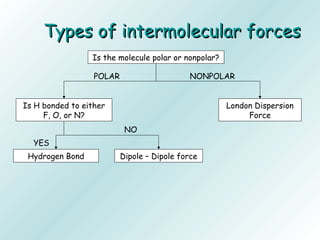
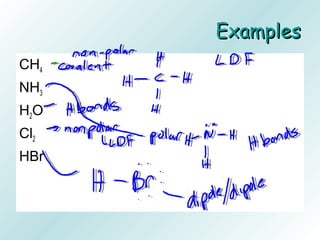
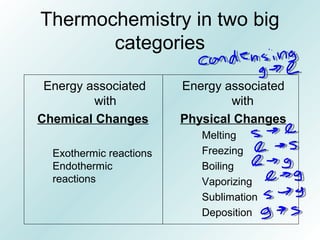
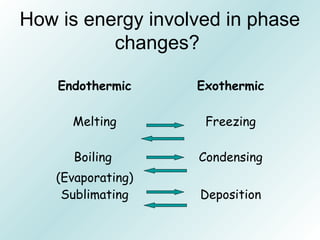
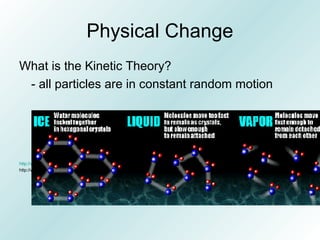
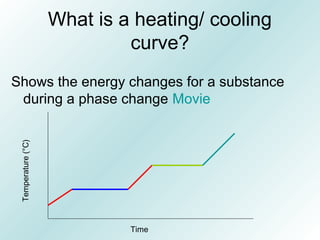
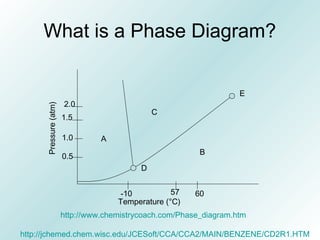
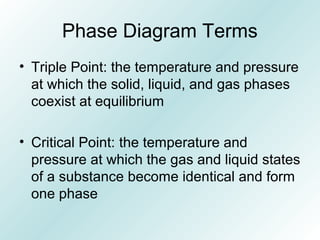
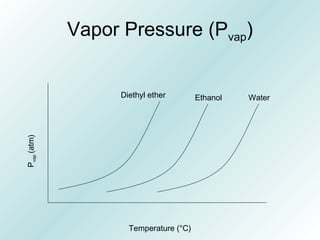
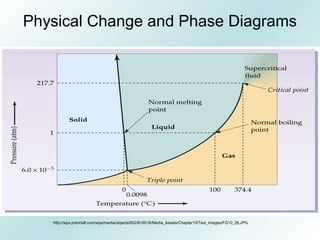
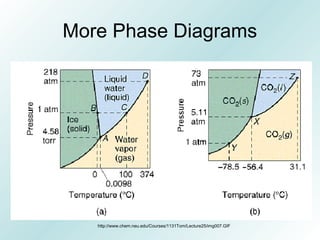
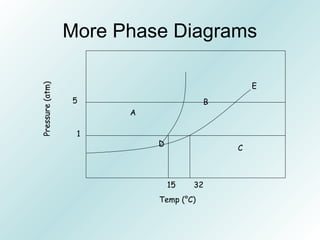
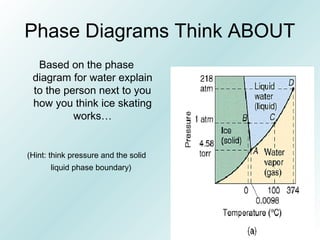
The document discusses liquids and solids from the perspective of the kinetic molecular theory. It explains that in liquids, particles are more closely packed than gases due to intermolecular forces, but are still able to flow freely unlike solids where particle motion is limited to vibration. Key differences between liquids and solids include density, compressibility, diffusion rates, and types of molecular structures (crystalline vs amorphous). The document also covers concepts such as surface tension, intermolecular forces including hydrogen bonding and London dispersion forces, and how force strength influences melting and boiling points.