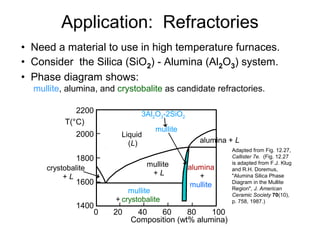
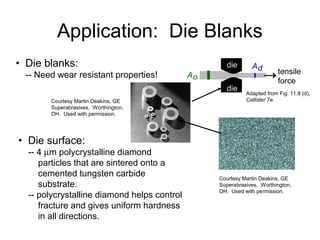
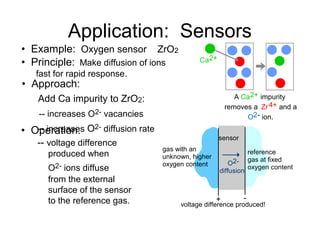
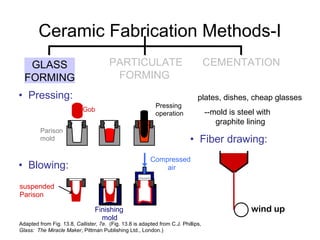
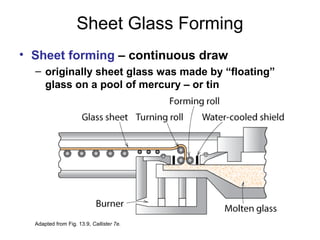
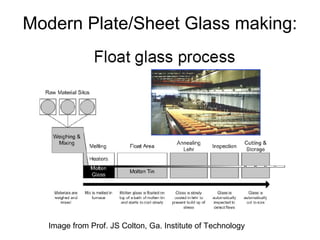
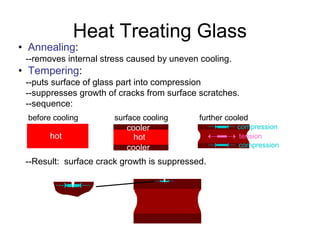
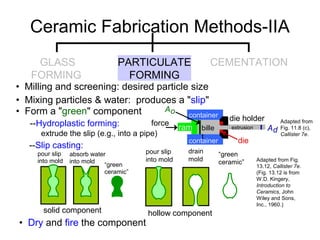
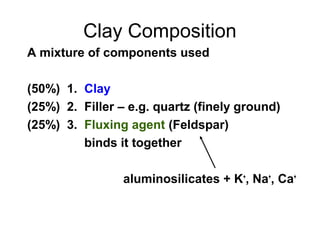
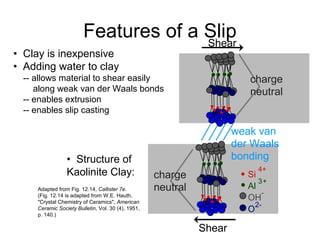
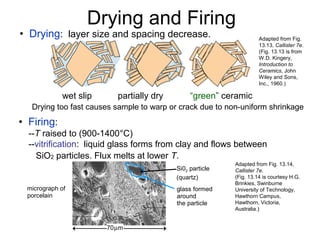
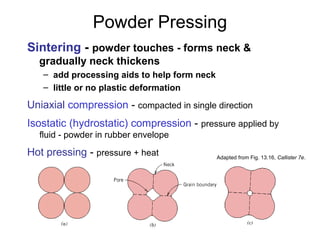
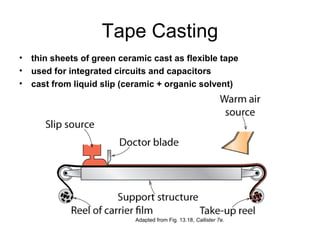
This document discusses ceramic properties, applications, and fabrication methods. It provides examples of using ceramics for refractories, die blanks, cutting tools, sensors, and alternative energy applications. Common ceramic fabrication techniques include glass forming, particulate forming, cementation, pressing, sintering, tape casting, and powder methods. Advanced ceramics have applications in heat engines, armor, and electronic packaging due to their high temperature resistance, hardness, and thermal conductivity.