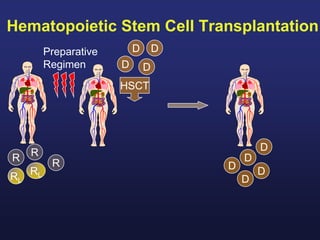
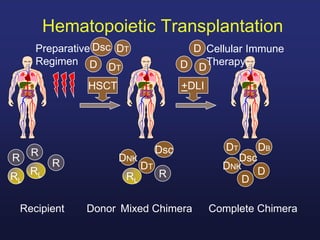
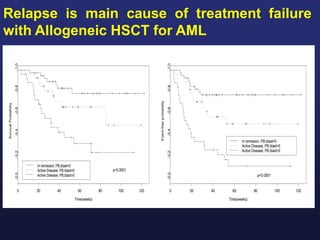
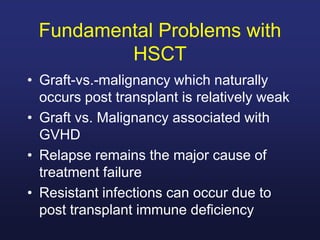
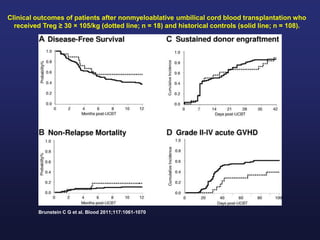
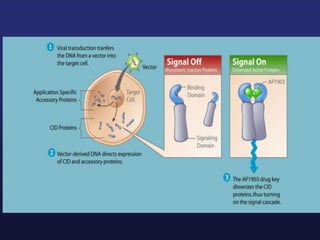
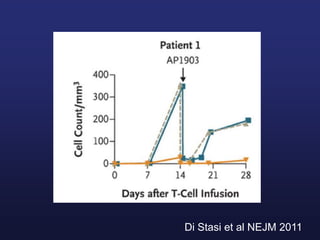
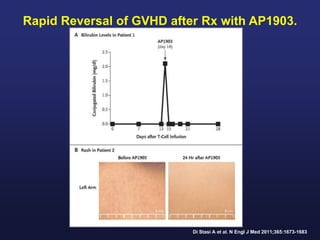
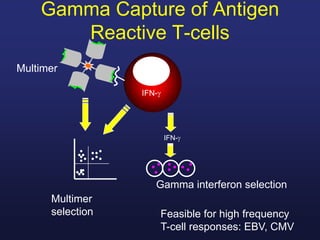
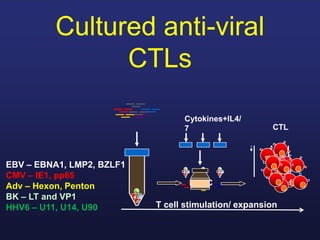
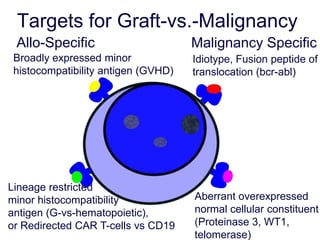
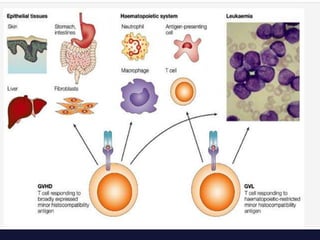
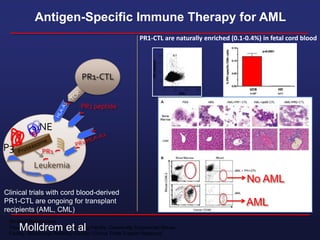
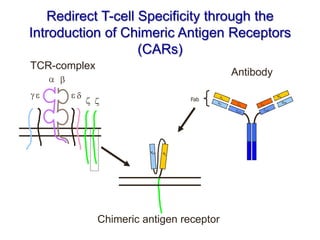
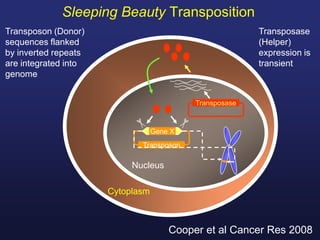
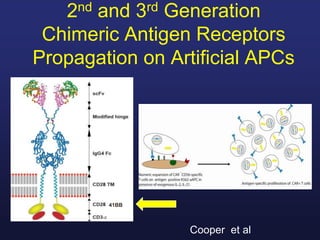
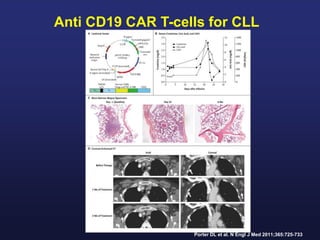
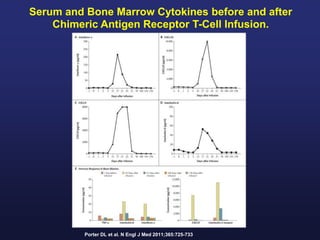
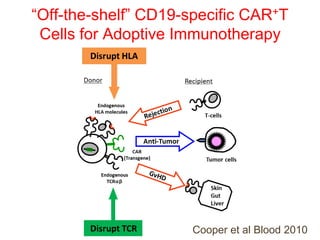
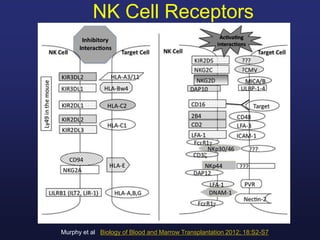
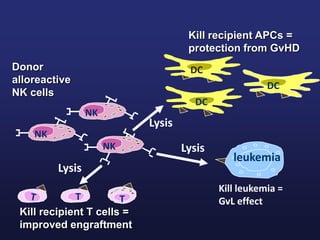
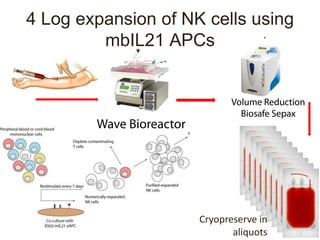
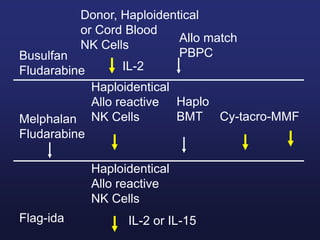
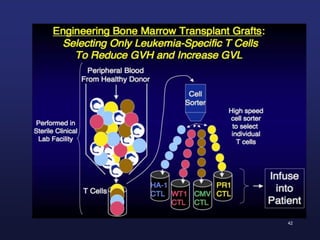
This document discusses cellular immune therapy approaches using hematopoietic stem cell transplantation. It notes that high-dose chemotherapy alone does not fully eradicate malignancies and relapse remains a major treatment failure. The graft-versus-malignancy effect provided by allogeneic transplantation is responsible for residual disease eradication but is associated with graft-versus-host disease. The document reviews approaches to enhance the graft-versus-malignancy effect through donor lymphocyte infusions, antigen-specific cytotoxic T-cells, and chimeric antigen receptor T-cells while preventing graft-versus-host disease using regulatory T-cells or genetically modified T-cells with suicide switches. Ongoing clinical trials