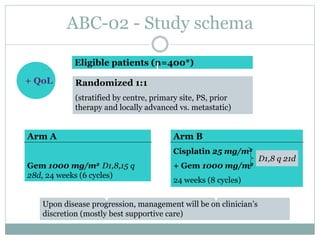
This document provides an overview of cholangiocarcinoma, a rare and deadly form of cancer. It discusses risk factors and increasing incidence rates. For localized disease, surgical resection is standard but outcomes remain poor. For advanced disease, gemcitabine-based chemotherapy is the standard first-line treatment based on results from the ABC-02 trial showing improved survival with gemcitabine and cisplatin. Retrospective data on second-line therapies and combination of pazopanib and trametinib show some benefit. Adding radiation therapy may also improve outcomes based on another retrospective review. Next generation sequencing is helping identify molecular alterations to guide targeted therapy trials. Ongoing clinical trials at MD Anderson include testing new