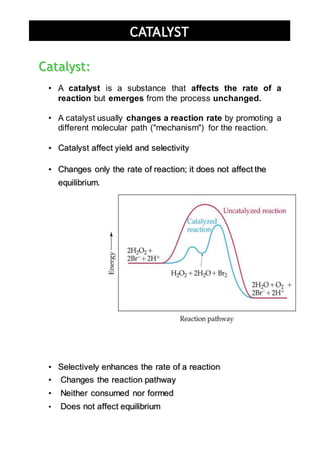
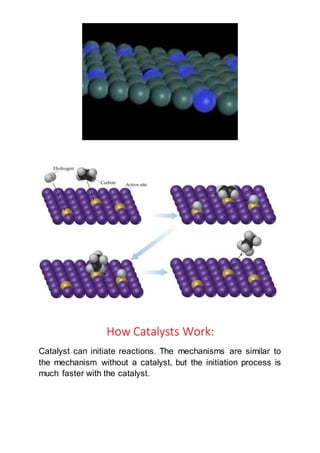
The document discusses catalysts and how they work. It defines a catalyst as a substance that speeds up a chemical reaction but remains unchanged after the reaction. It states that catalysts lower the activation energy of reactions, allowing more effective collisions between reactant molecules. The document distinguishes between homogeneous and heterogeneous catalysts, providing examples of each type. It explains that catalysts can work by initiating reactions through faster intermediate steps or by stabilizing reactive intermediates.