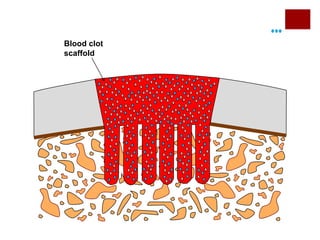
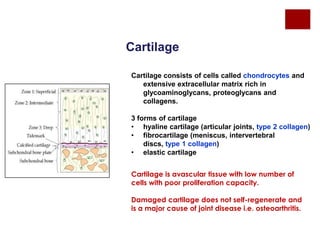
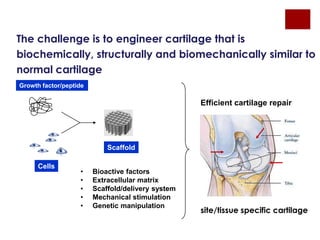
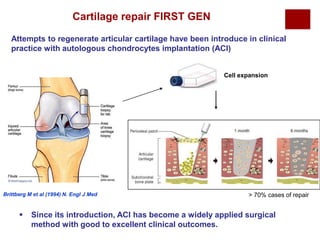
The document discusses the challenges of cartilage regeneration in osteoarthritis (OA), detailing the types of cartilage and the difficulties in repairing damaged cartilage due to its low cell proliferation capacity. It explores various cellular therapies including adult chondrocytes, mesenchymal stem cells, and embryonic stem cells, highlighting the promising potential of these methods despite existing limitations such as availability and ethical concerns. Ultimately, the document underscores the urgent need for effective strategies to repair articular cartilage damage and improve joint function for OA patients.




![Adult Mesenchymal stem
cell based therapy
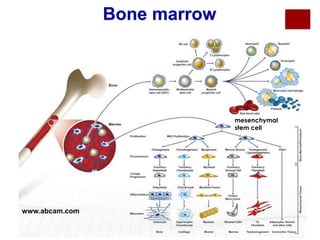
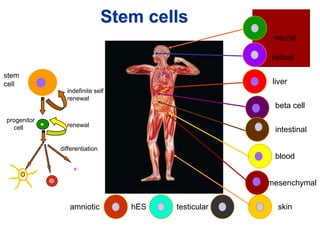
Mesenchymal Stem Cells (MSC) can be
obtained from bone marrow, synovium, fat, or
umbilical cord.
Distinct potential for differentiation into the
chondrogenic lineage.
Adipocyte-derived MSC have been suggested to
be most chondrogenic
May be differentiated into chondrocytes using
growth factors such as (BMP) (IGF) (FGF], or using
scaffolds [40] which may release prochondrogenic signals such as TGF-β](https://image.slidesharecdn.com/cartilagerepairbagaria-140201033539-phpapp02/85/Cartilage-Repair-using-Stem-cell-Orthobiologics-11-320.jpg)