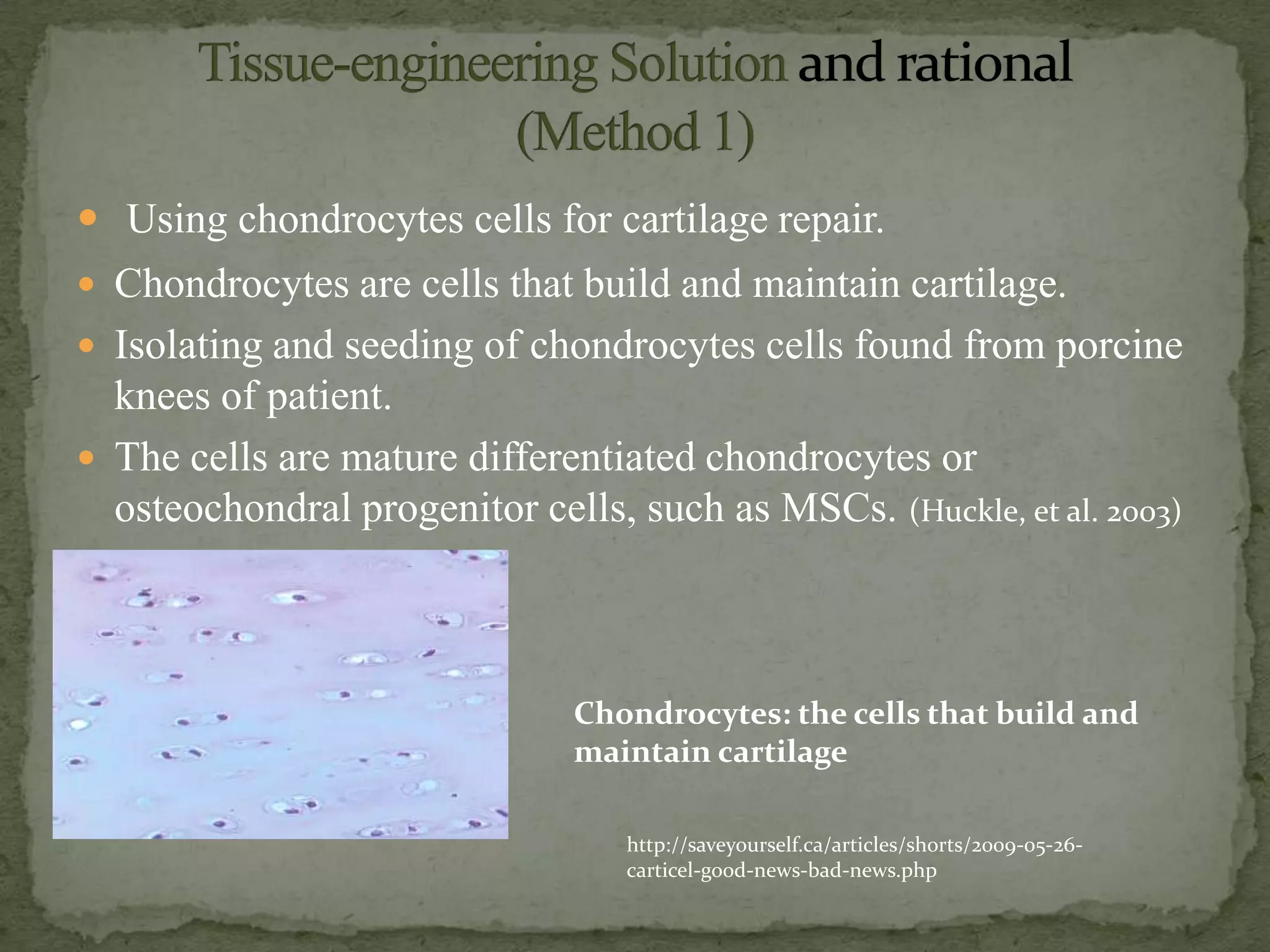
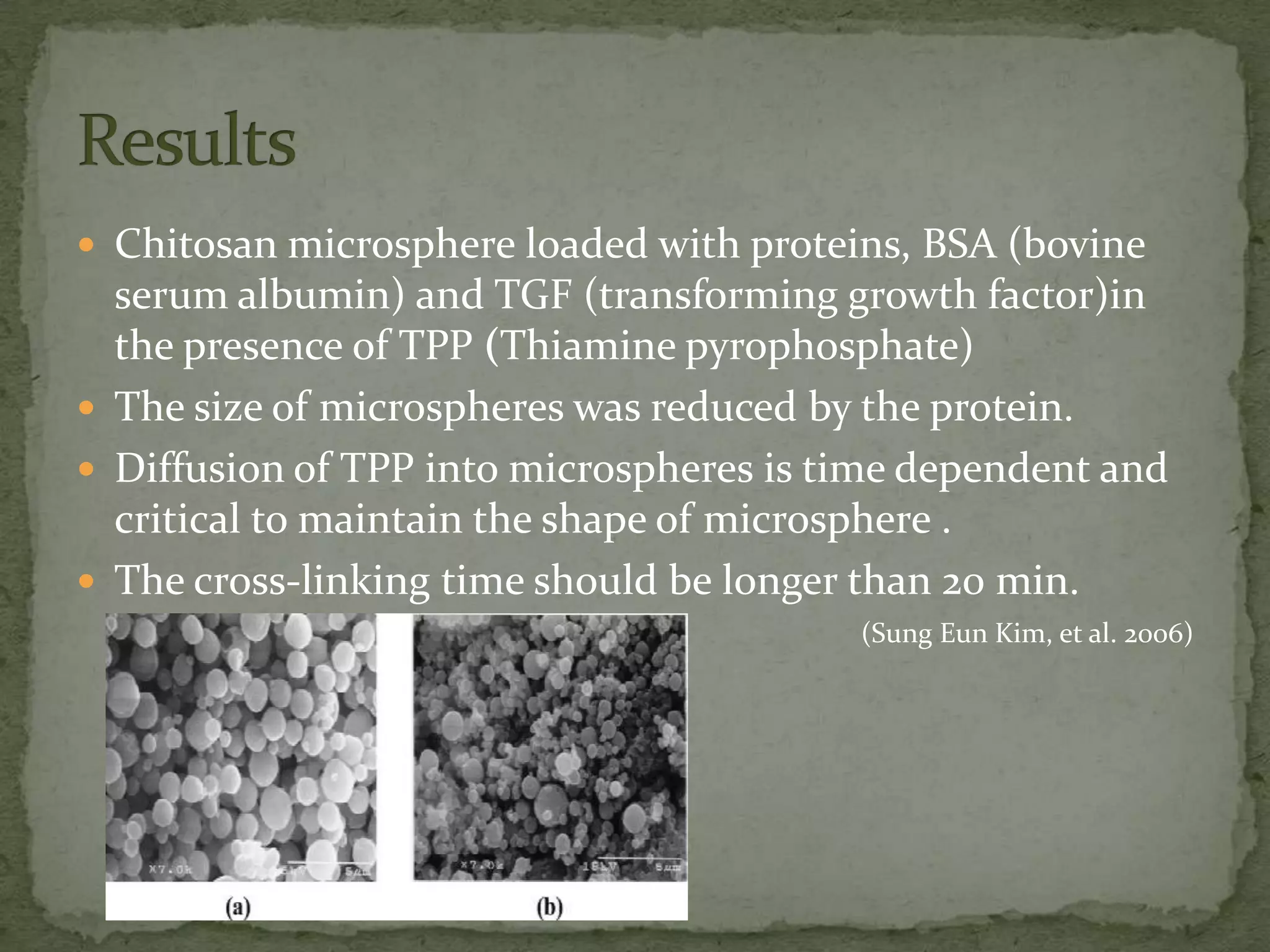
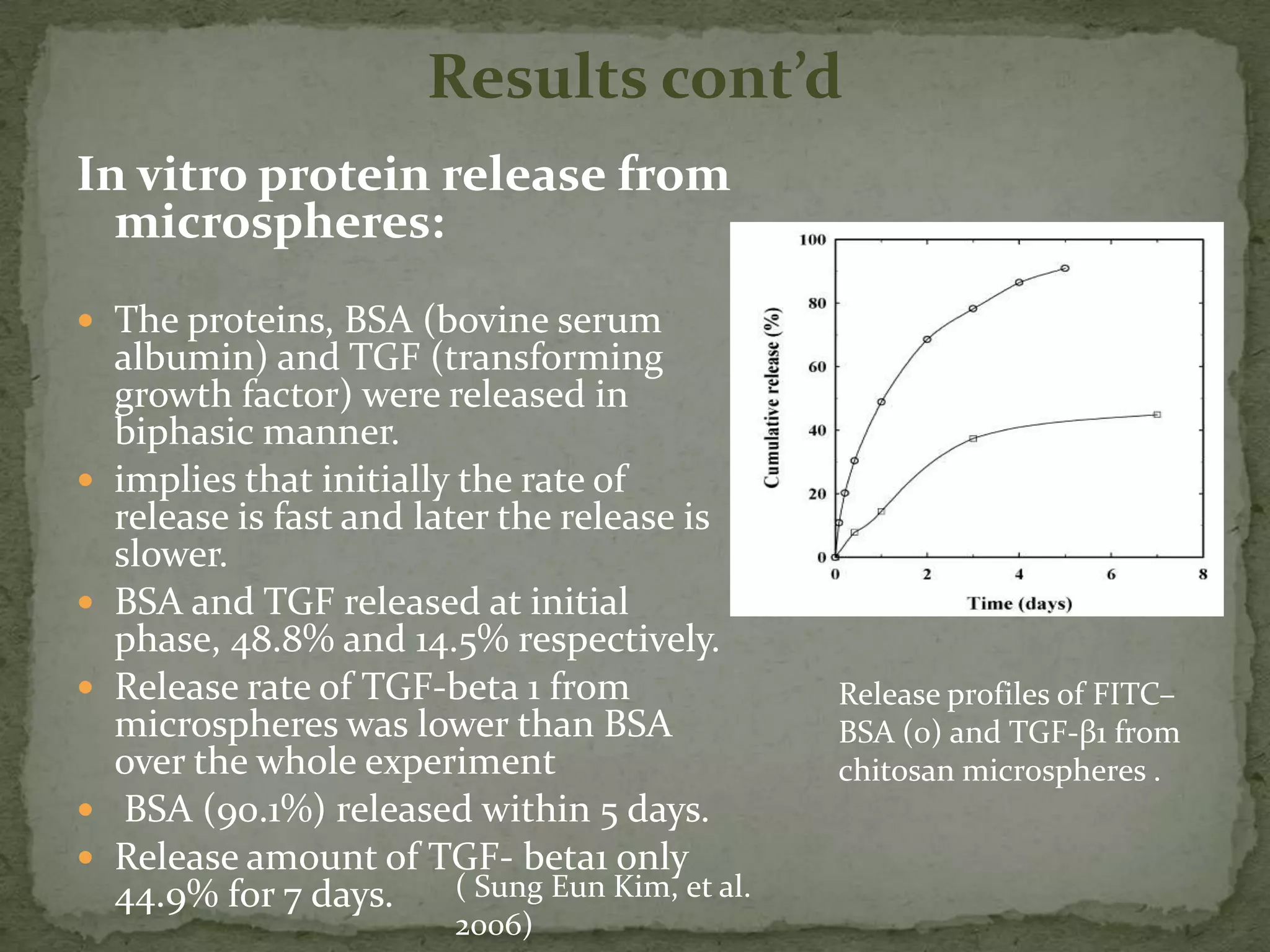
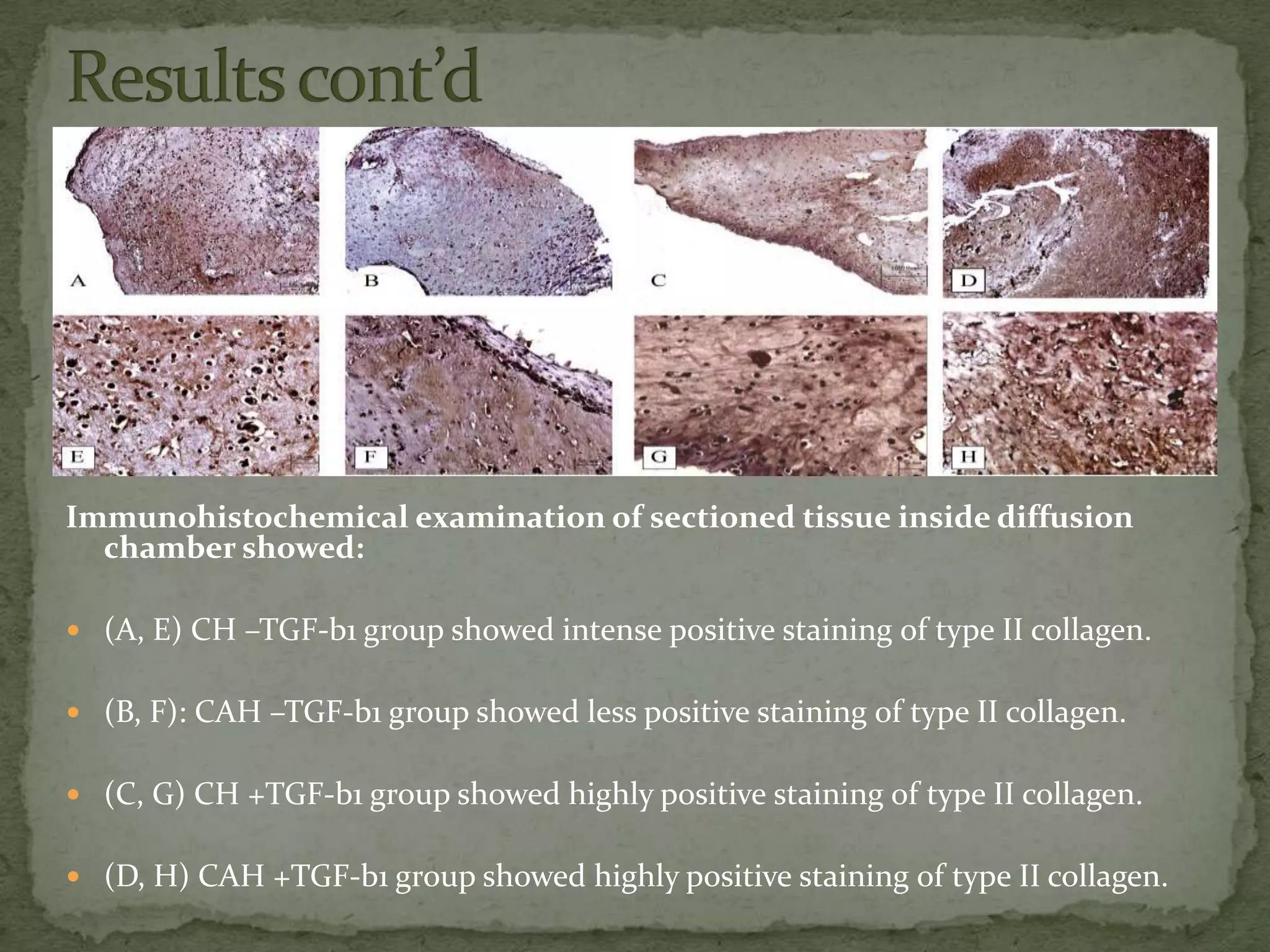
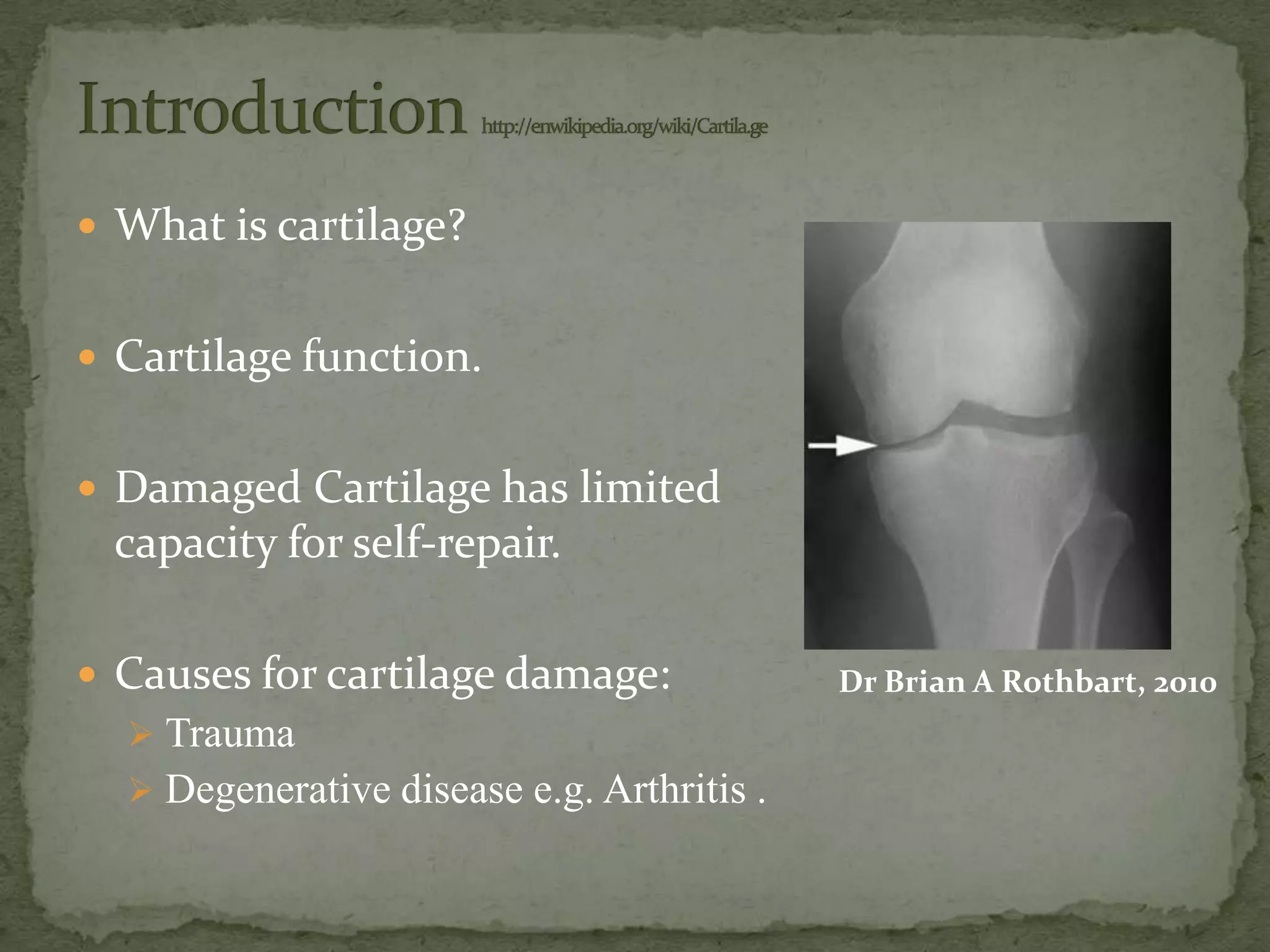
This document discusses cartilage damage and potential treatments using tissue engineering. It describes how cartilage has limited ability for self-repair and can be damaged by trauma or arthritis. Two potential treatments are discussed: (1) Isolating and seeding chondrocytes cells on scaffolds to repair cartilage, and (2) Using mesenchymal stem cells (MSCs) that can differentiate into chondrocytes when implanted on scaffolds. The document evaluates studies comparing the effectiveness of these two approaches for cartilage regeneration and repair.


![ 17.1% of the disabilities in USA because of arthritis.
[Healthy people]
350 million people
worldwide have arthritis.
37 million people in USA.
wrongdiagnosis.com
Health care: $81 billion annually in USA. abcnews.go.com](https://image.slidesharecdn.com/652finalpresentation-13416721602532-phpapp01-120707094559-phpapp01/75/Cartilage-Tissue-Engineering-4-2048.jpg)