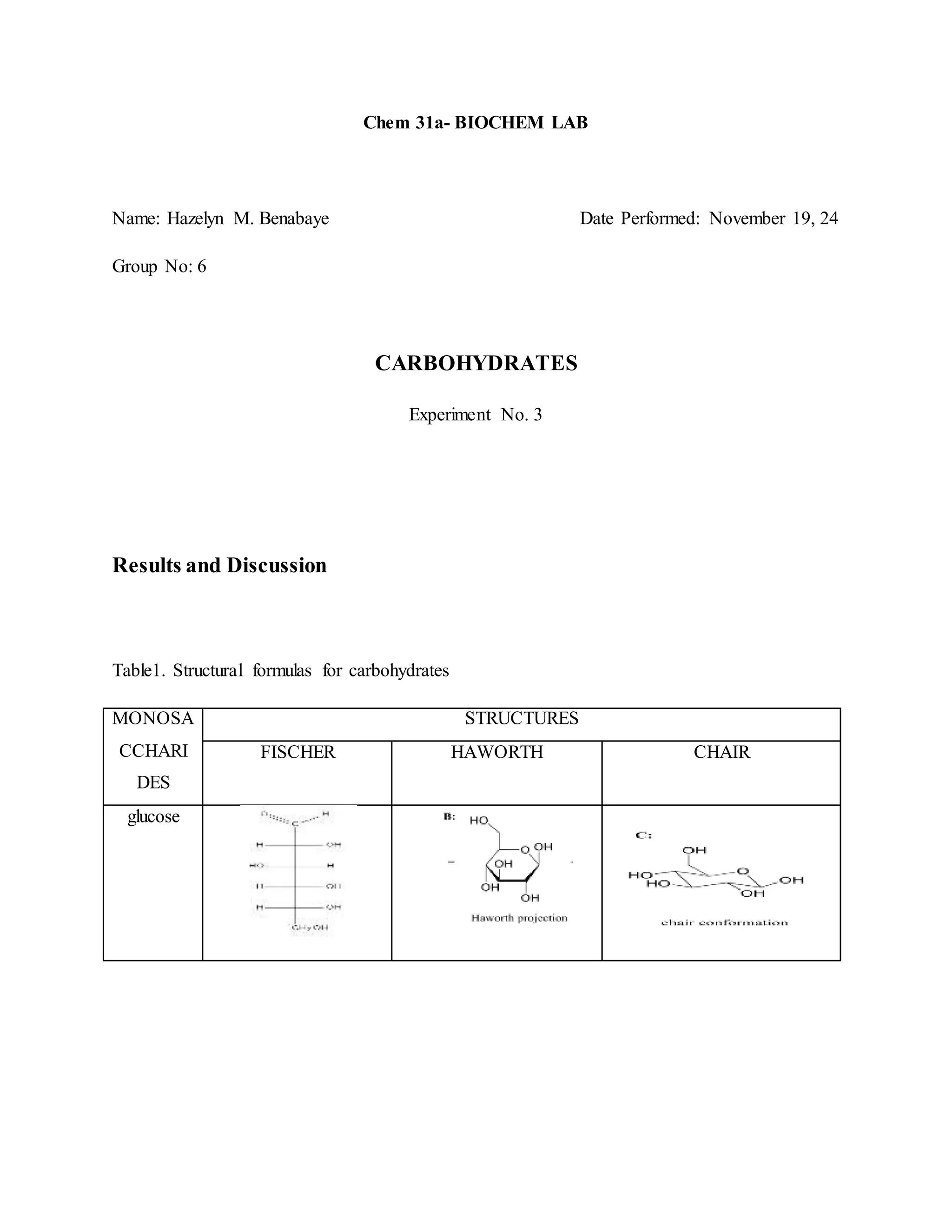
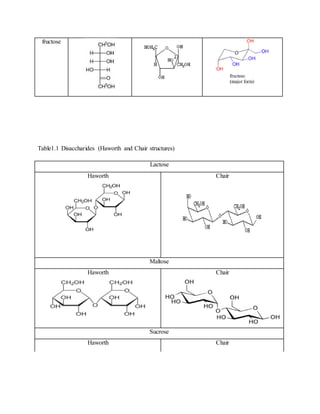
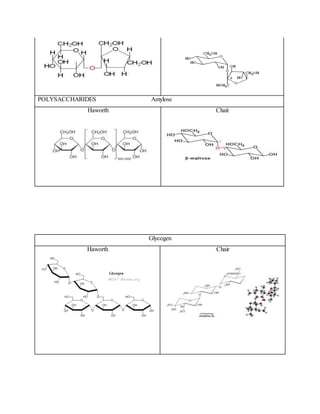
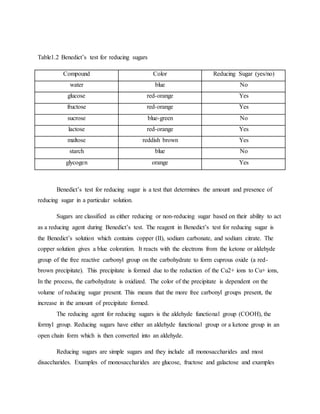
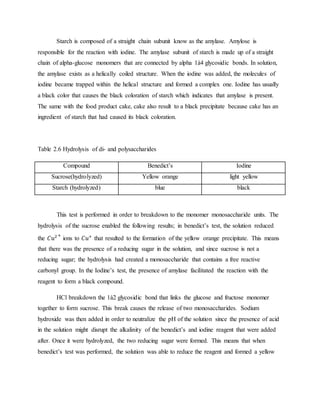
1. The document reports on the results of several tests performed on carbohydrates to identify their structure and type. Benedict's test, Barfoed's test, Seliwanoff's test, and iodine tests were used to classify samples as reducing sugars, monosaccharides, ketohexoses, and polysaccharides. 2. Key results showed that glucose and fructose reacted positively in most tests, identifying them as reducing monosaccharides. Sucrose did not reduce before hydrolysis but did after, when it broke down into glucose and fructose. 3. Starch strongly reacted with iodine, identifying it as a polysaccharide. Overall, the tests helped characterize the carbohydrate samples