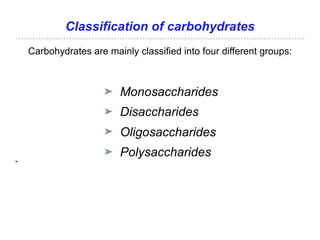
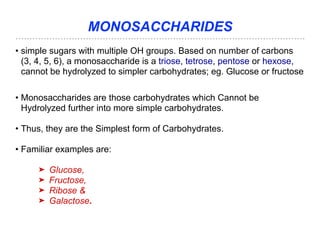
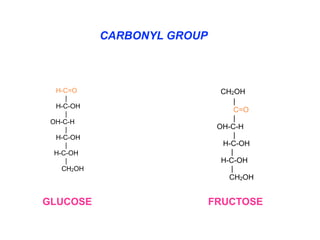
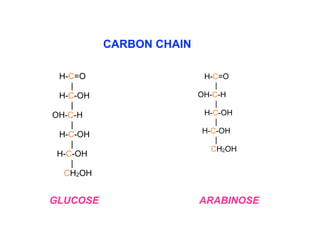
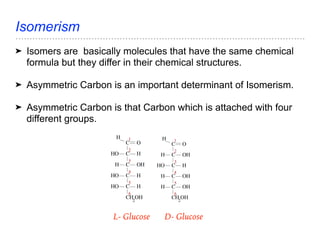
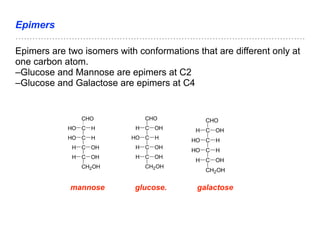
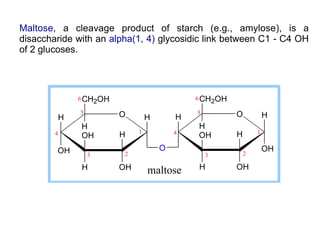
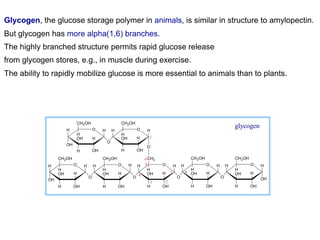
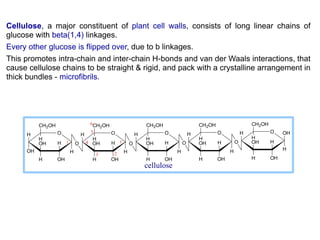
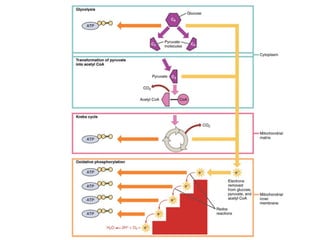
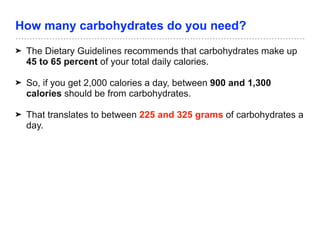
The document discusses the role of carbohydrates in health, metabolism, and nutrition, emphasizing their importance for energy, immunity, and cognitive function. It categorizes carbohydrates into monosaccharides, disaccharides, oligosaccharides, and polysaccharides while outlining processes like digestion, absorption, and metabolism. It also highlights dietary guidelines for carbohydrate intake and mentions disorders related to carbohydrate metabolism.









![What are CARBOHYDRATES ?
–Are hydrated carbon molecules [CnH2nOn or (CH2O)n],
–They are virtually ubiquitous because they have such a wide
range of structures and functions
Structure:
–polyhydroxylated ketones,
–polyhydroxylated aldehydes, or
–compounds that can be hydrolyzed into these compounds.](https://image.slidesharecdn.com/chempresentation-201208074707/85/Carbohydrates-10-320.jpg)