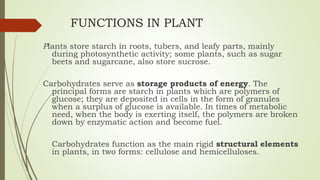
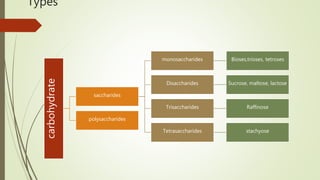
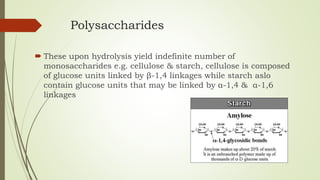
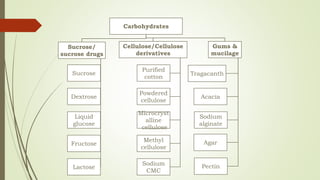
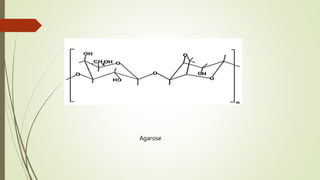
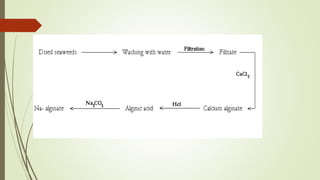
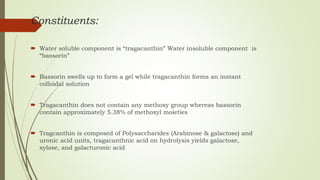
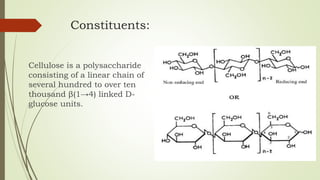
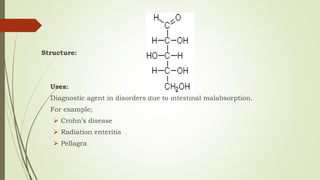
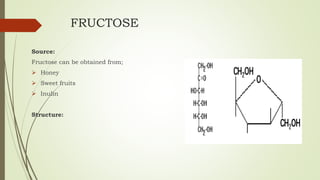
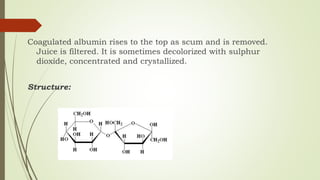
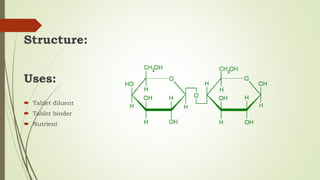
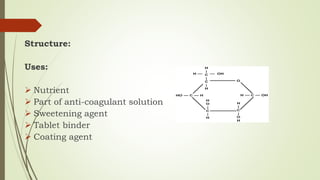
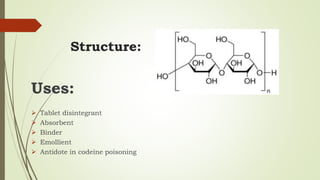
Carbohydrates are organic compounds made primarily of carbon, hydrogen, and oxygen, functioning mainly as energy storage and structural elements in plants. They can be classified into monosaccharides, disaccharides, and polysaccharides, with various tests available to identify their presence. Additionally, carbohydrates are significant in both pharmacognosy and culinary applications, found in substances like gums and mucilages, agar, and pectin, each serving unique health and industrial purposes.