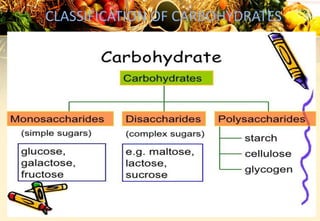
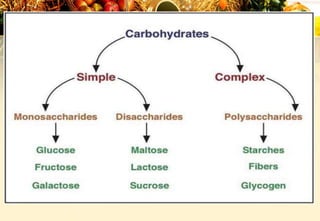
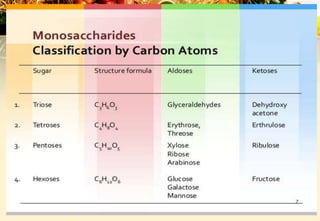
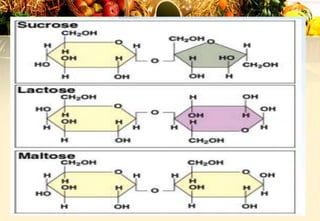
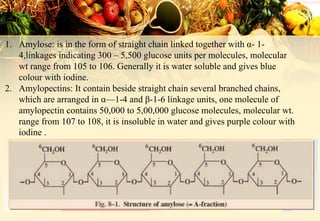
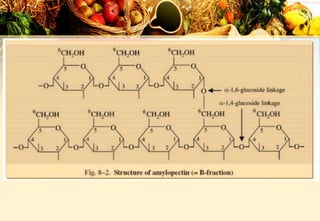
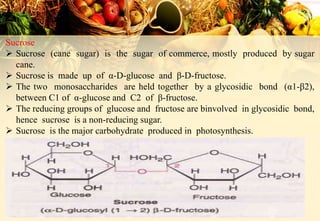
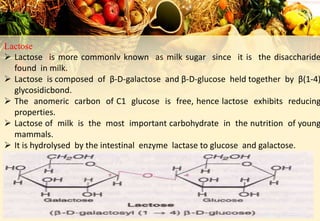
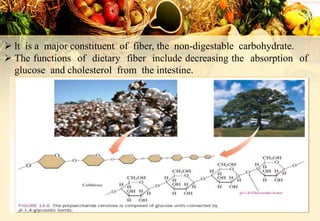
Carbohydrates are organic compounds made of carbon, hydrogen, and oxygen that provide energy. They are classified as monosaccharides, oligosaccharides, or polysaccharides based on their size. The main carbohydrates in food are starch, sucrose, lactose, and fiber. Carbohydrates are important for energy, structure, nutrient storage, and have many health benefits, but excess sugar intake can lead to issues like tooth decay, obesity, and diabetes.