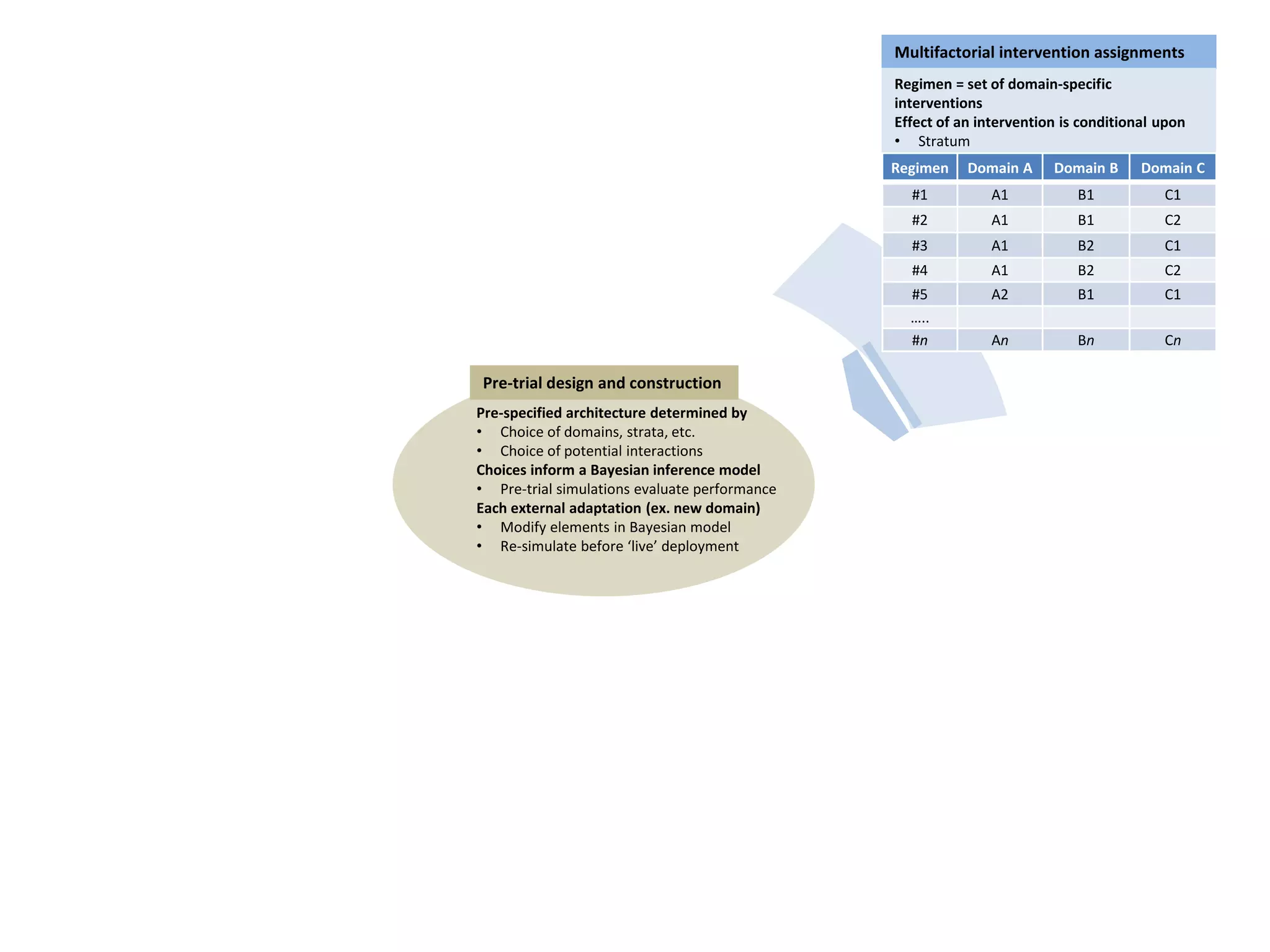
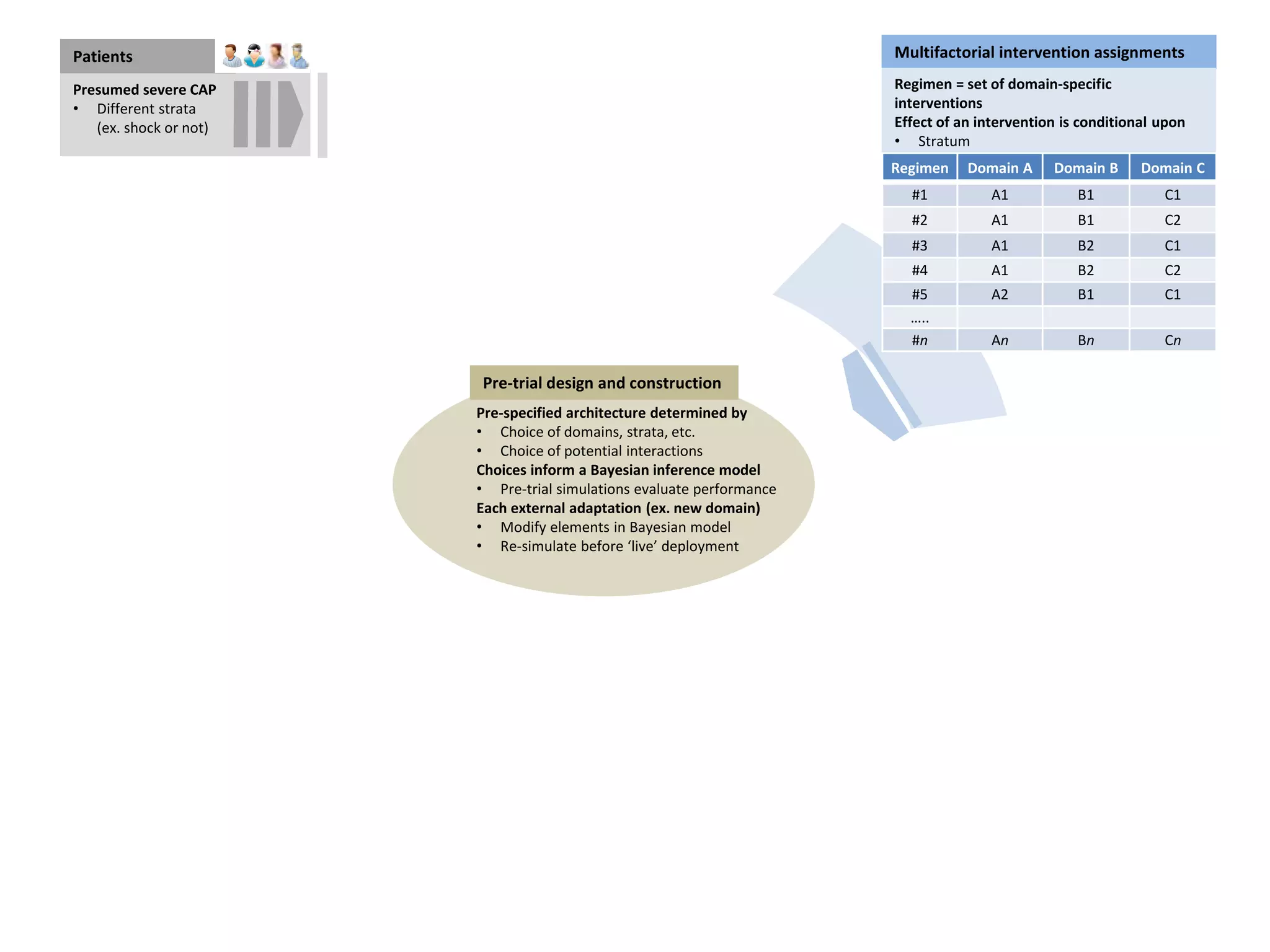
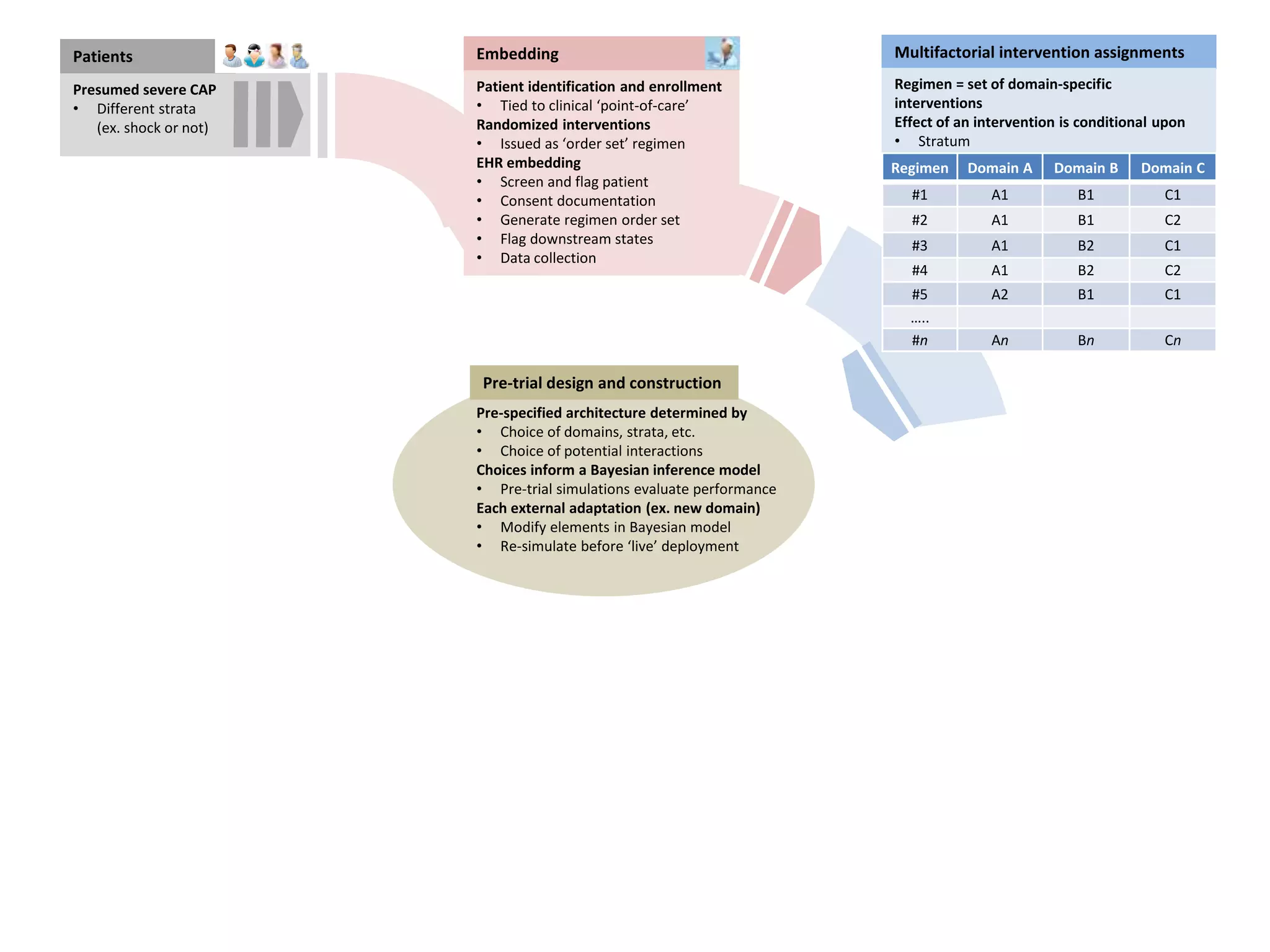
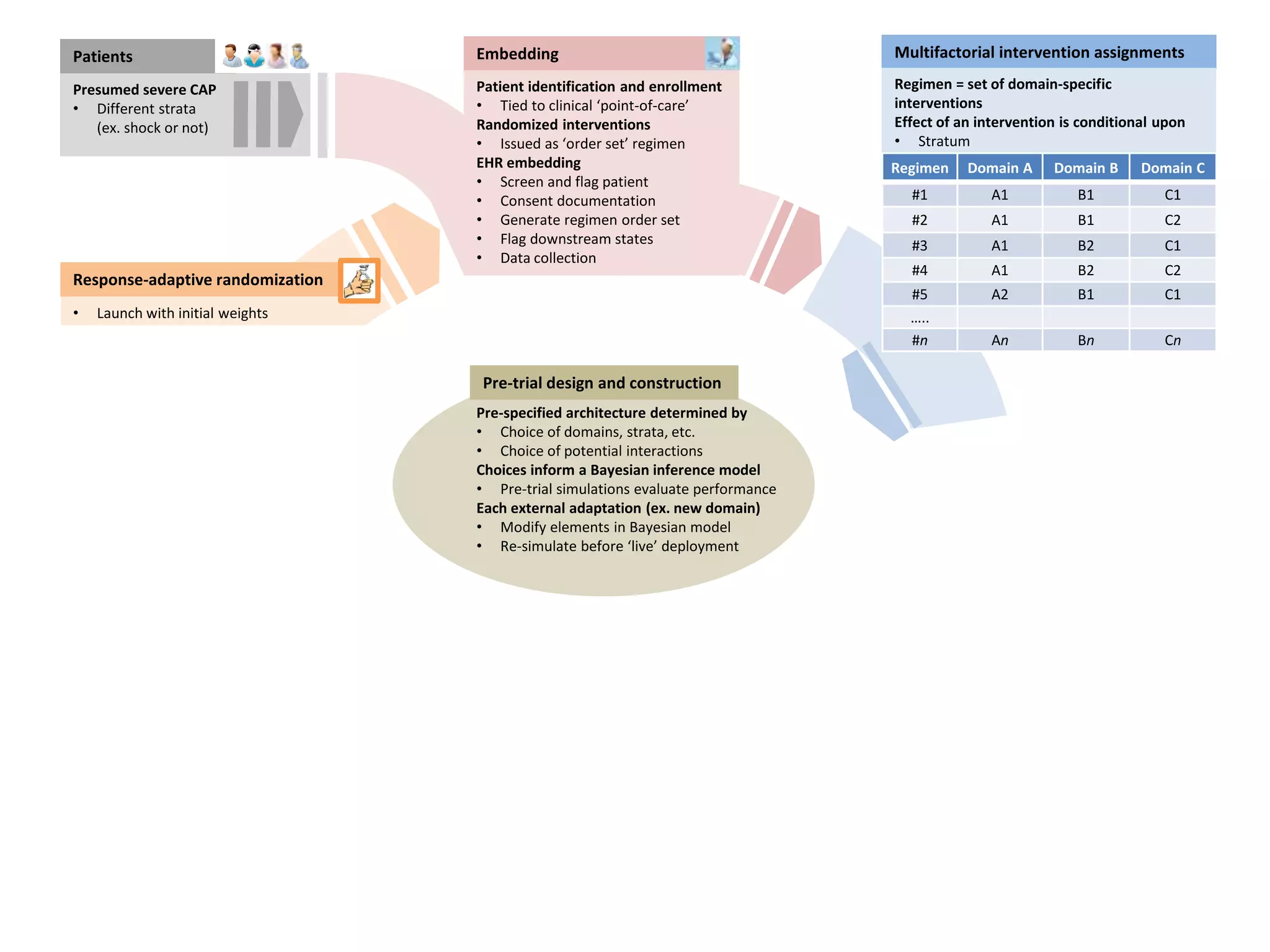
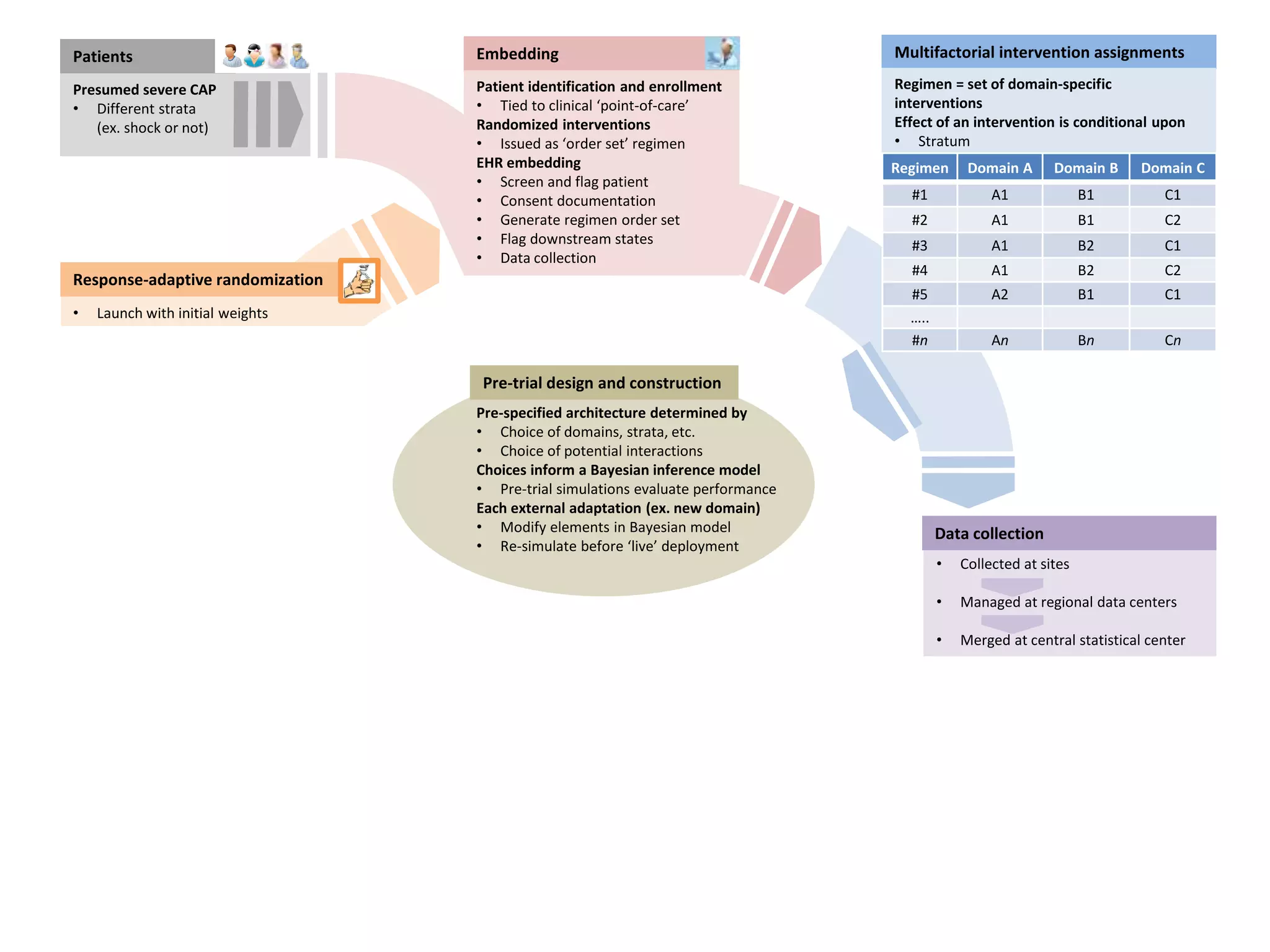
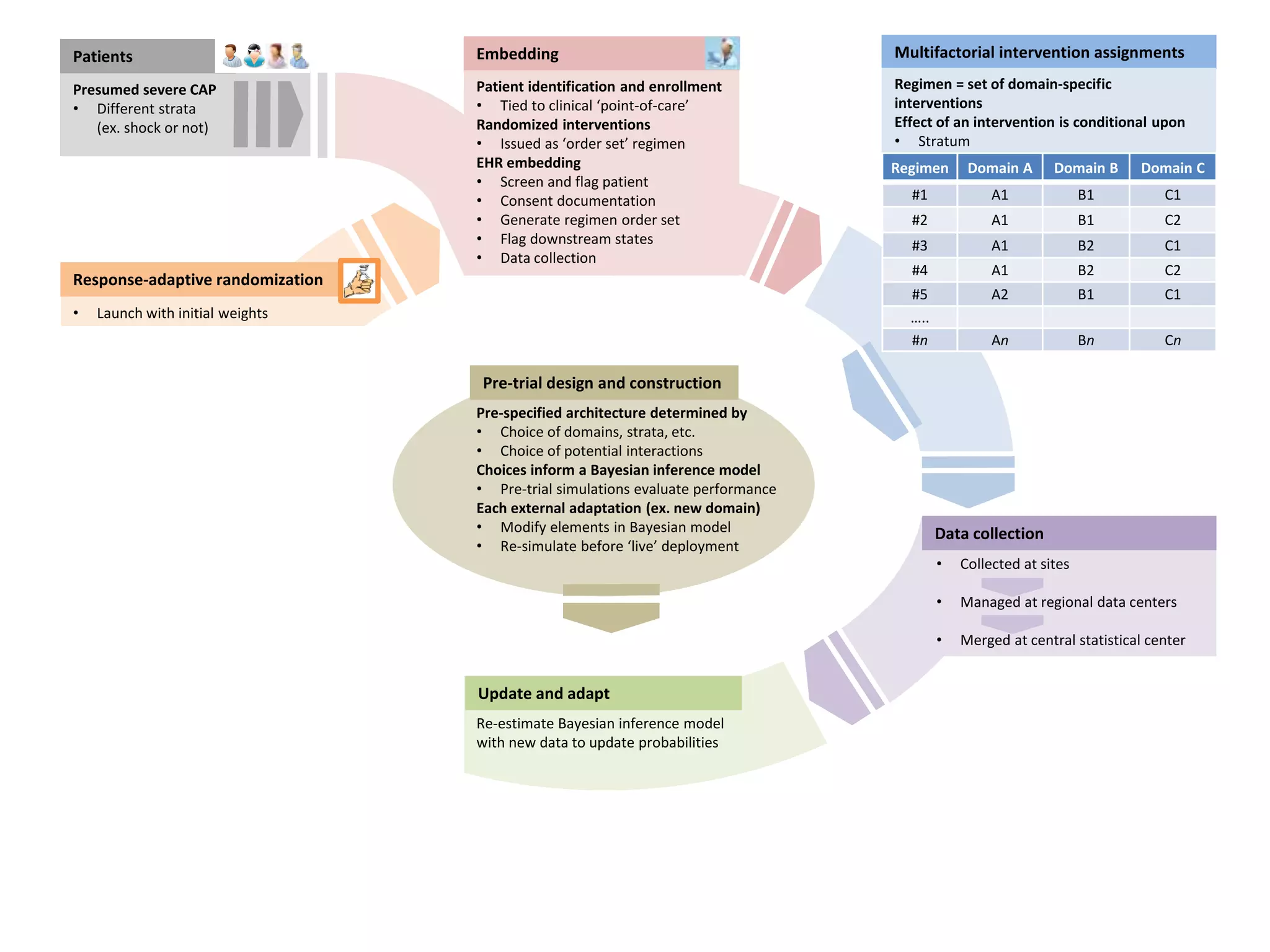
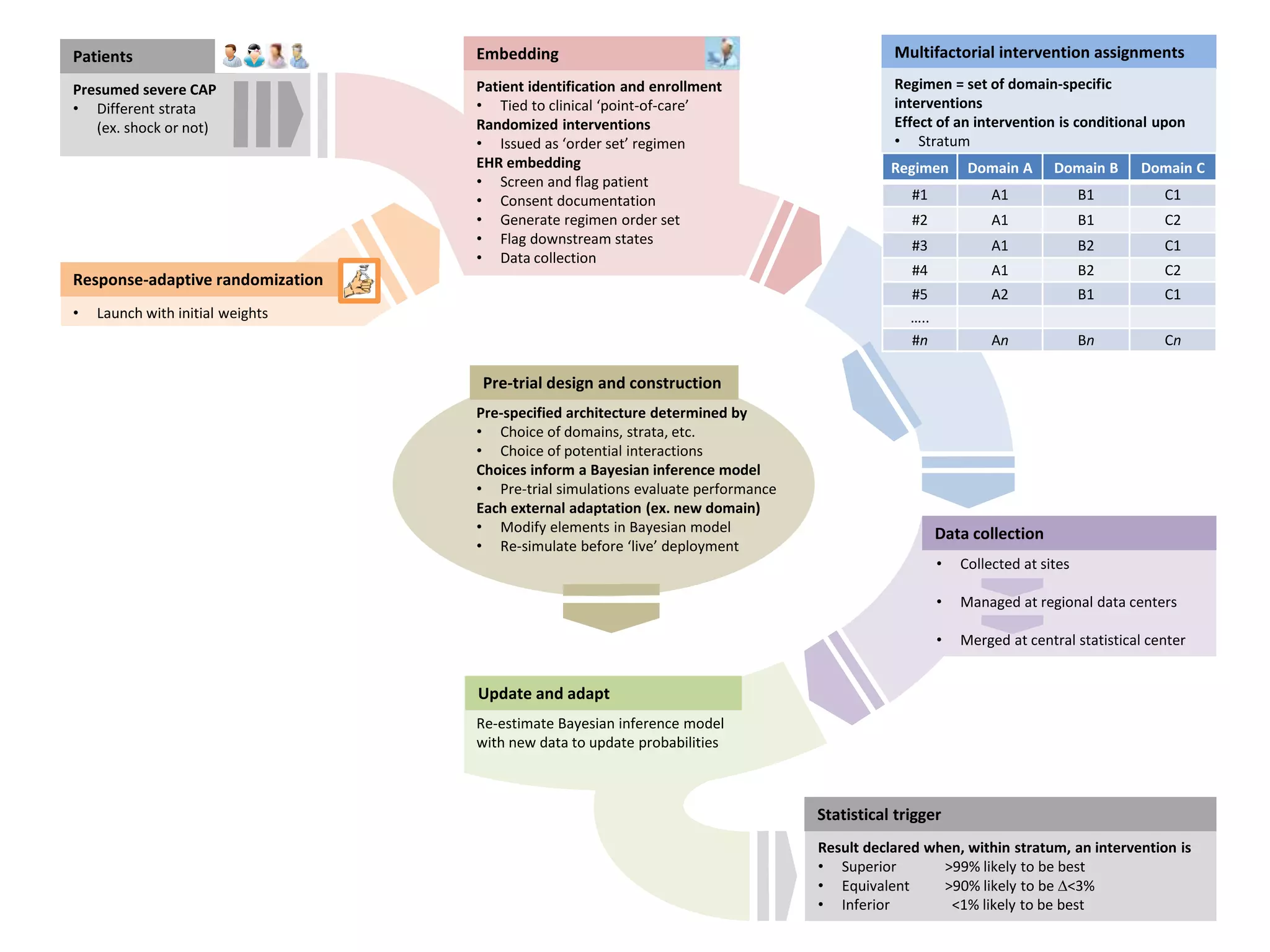
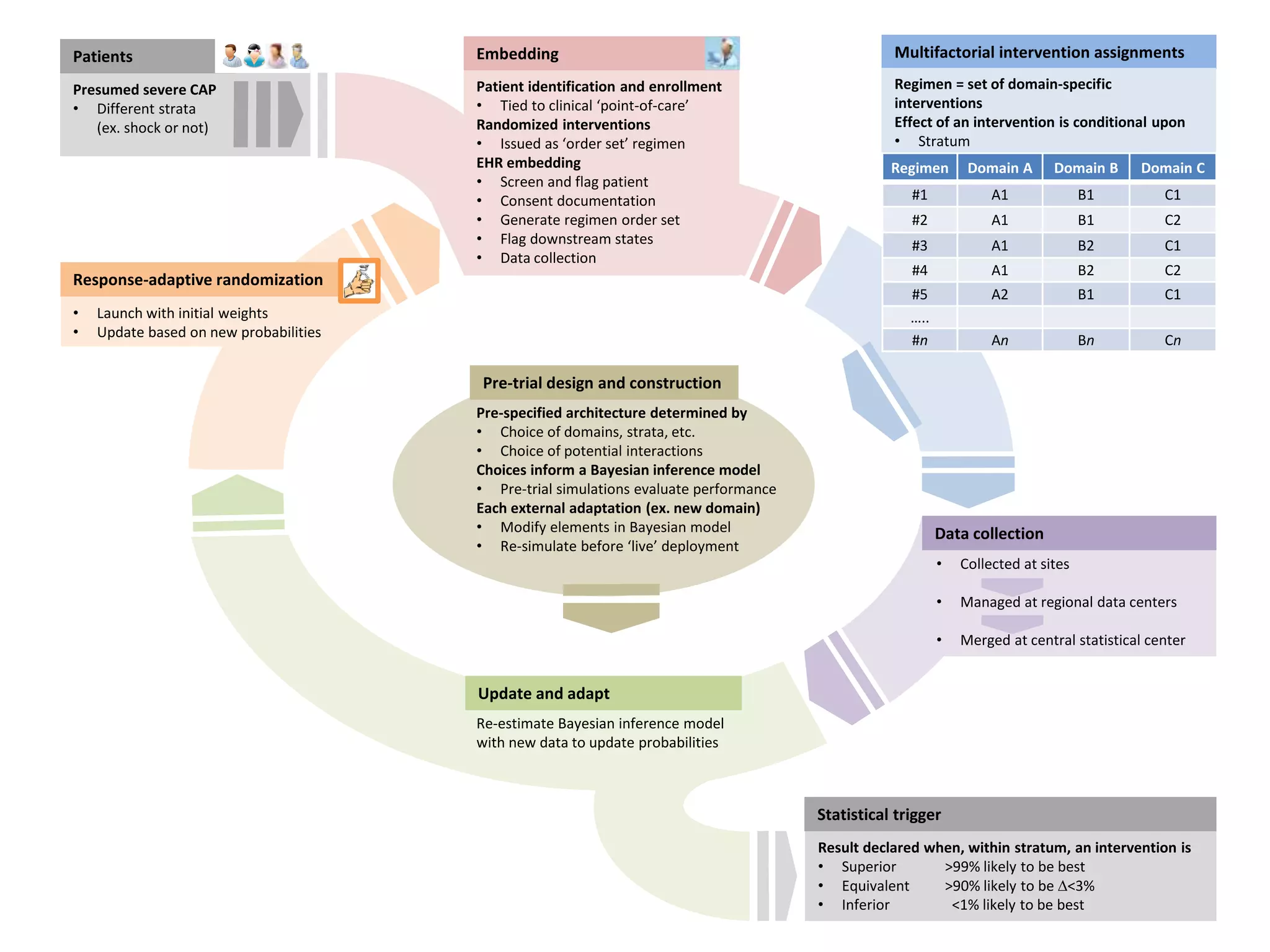
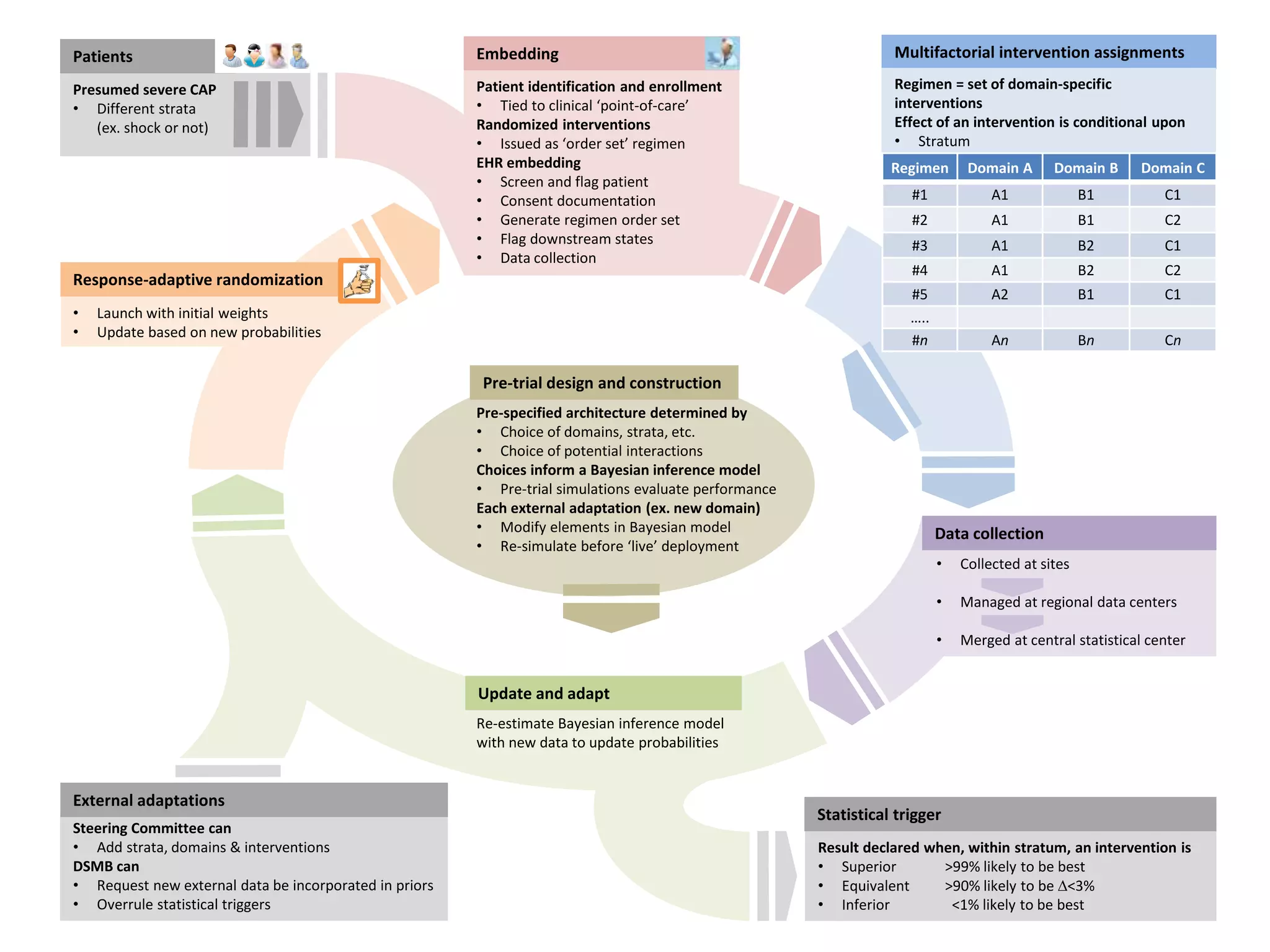
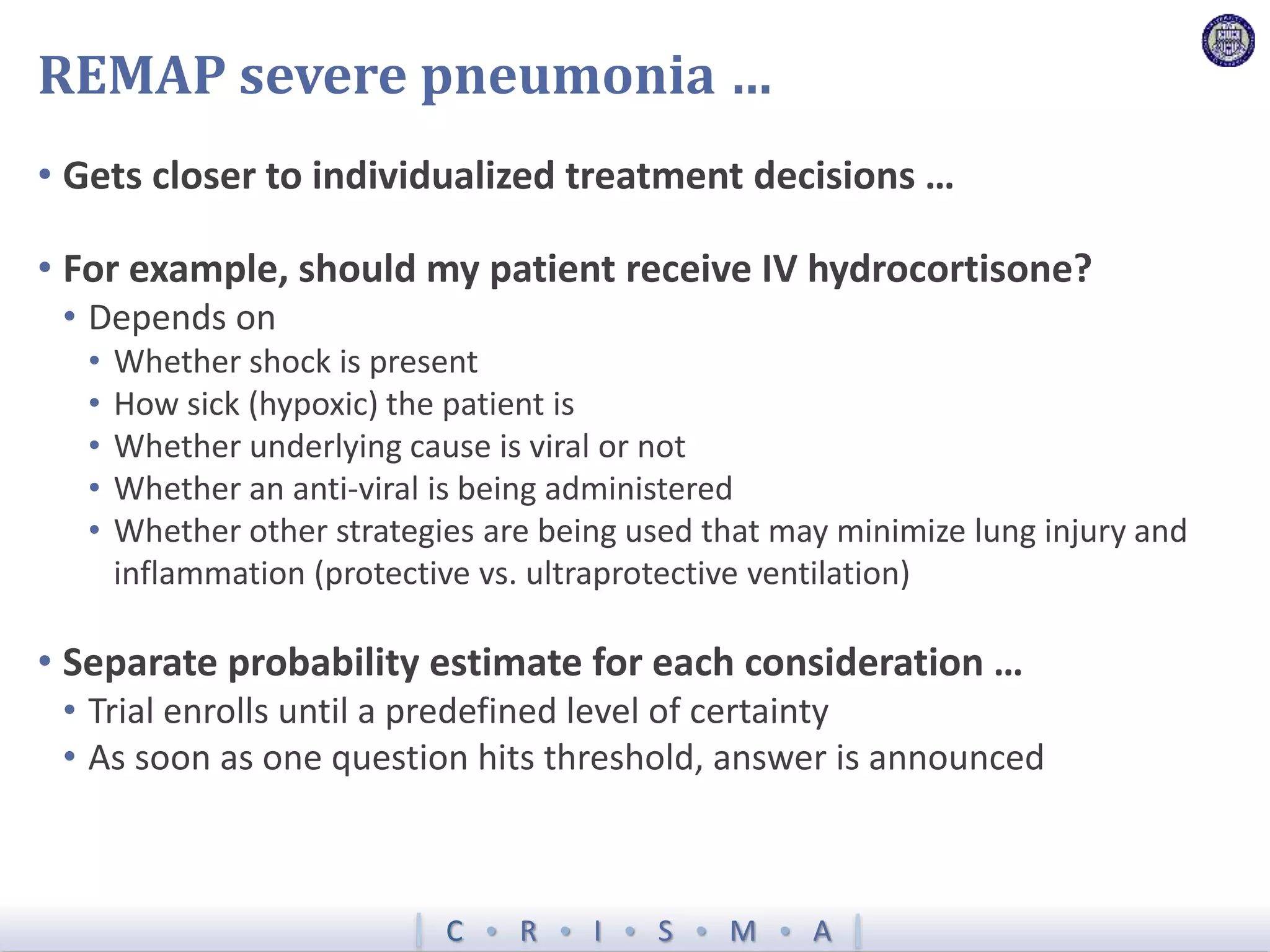
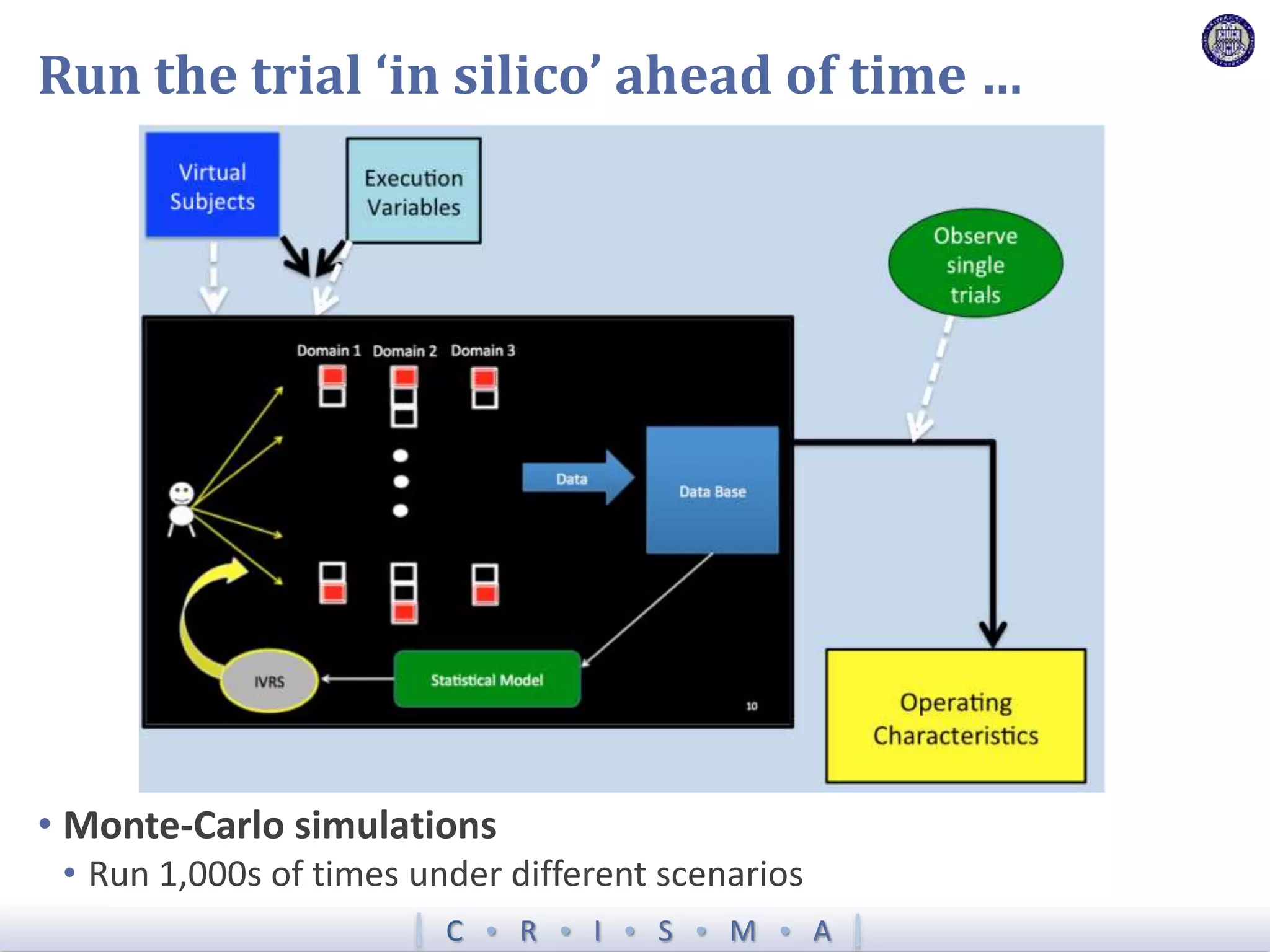
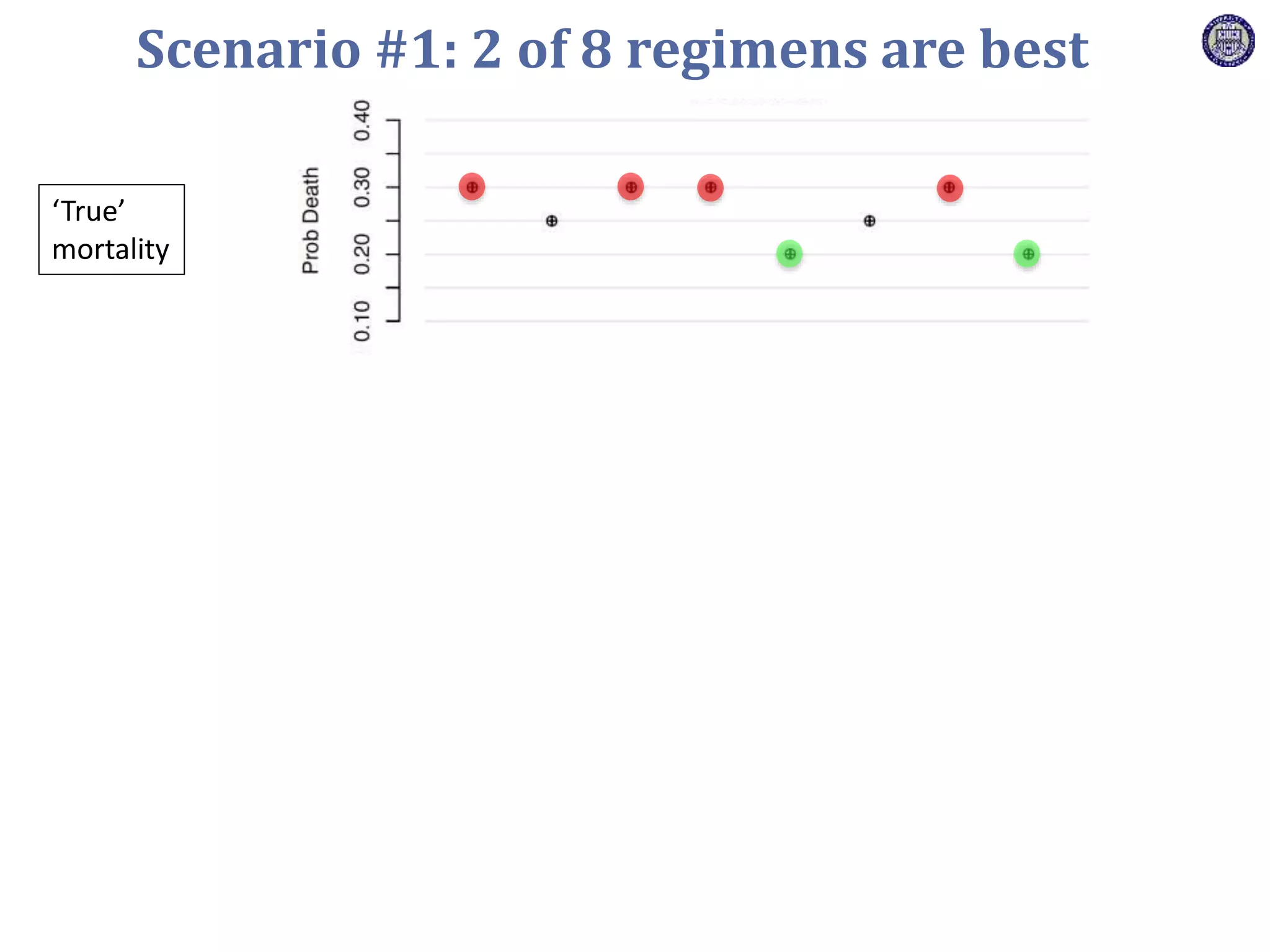
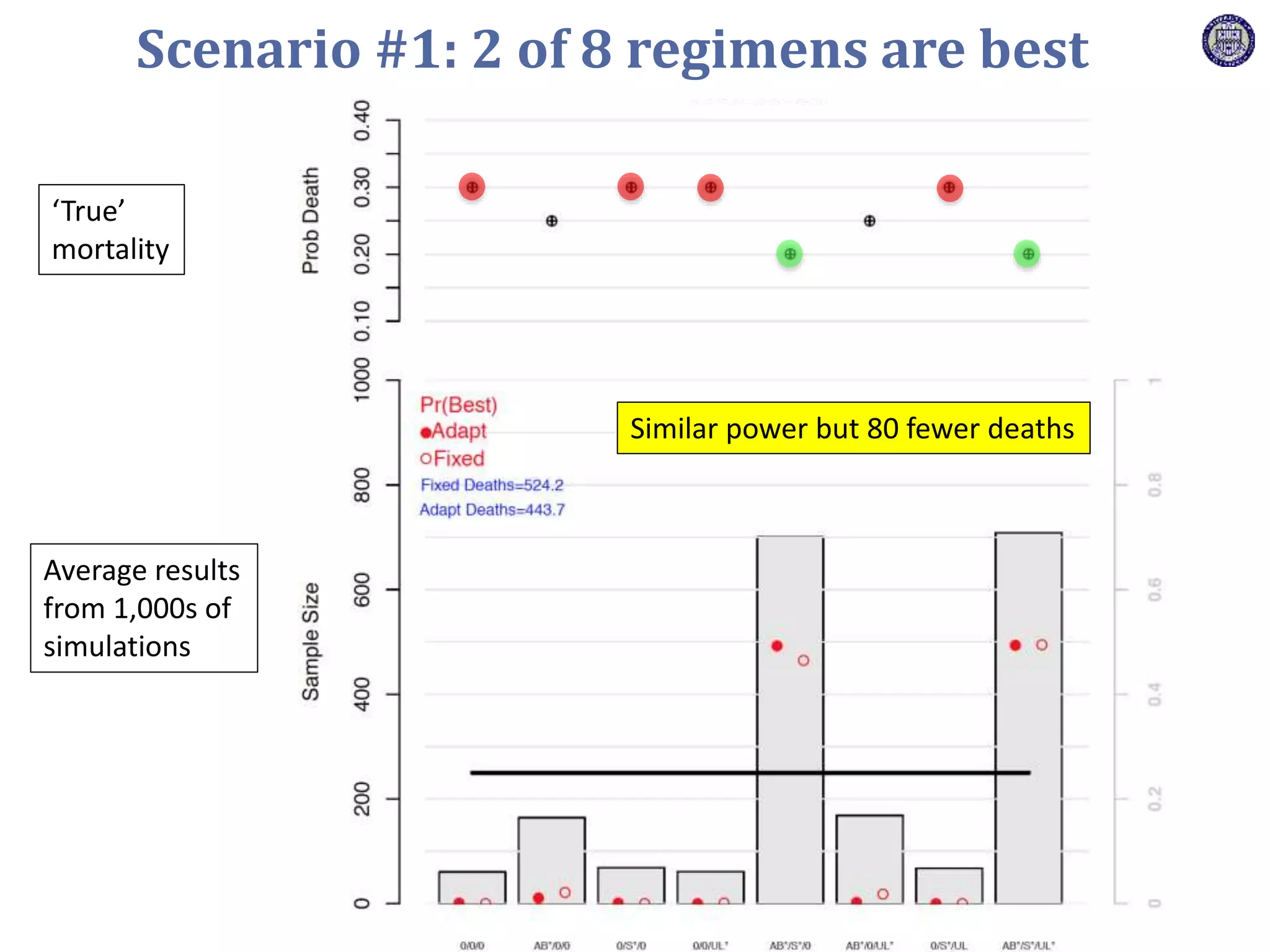
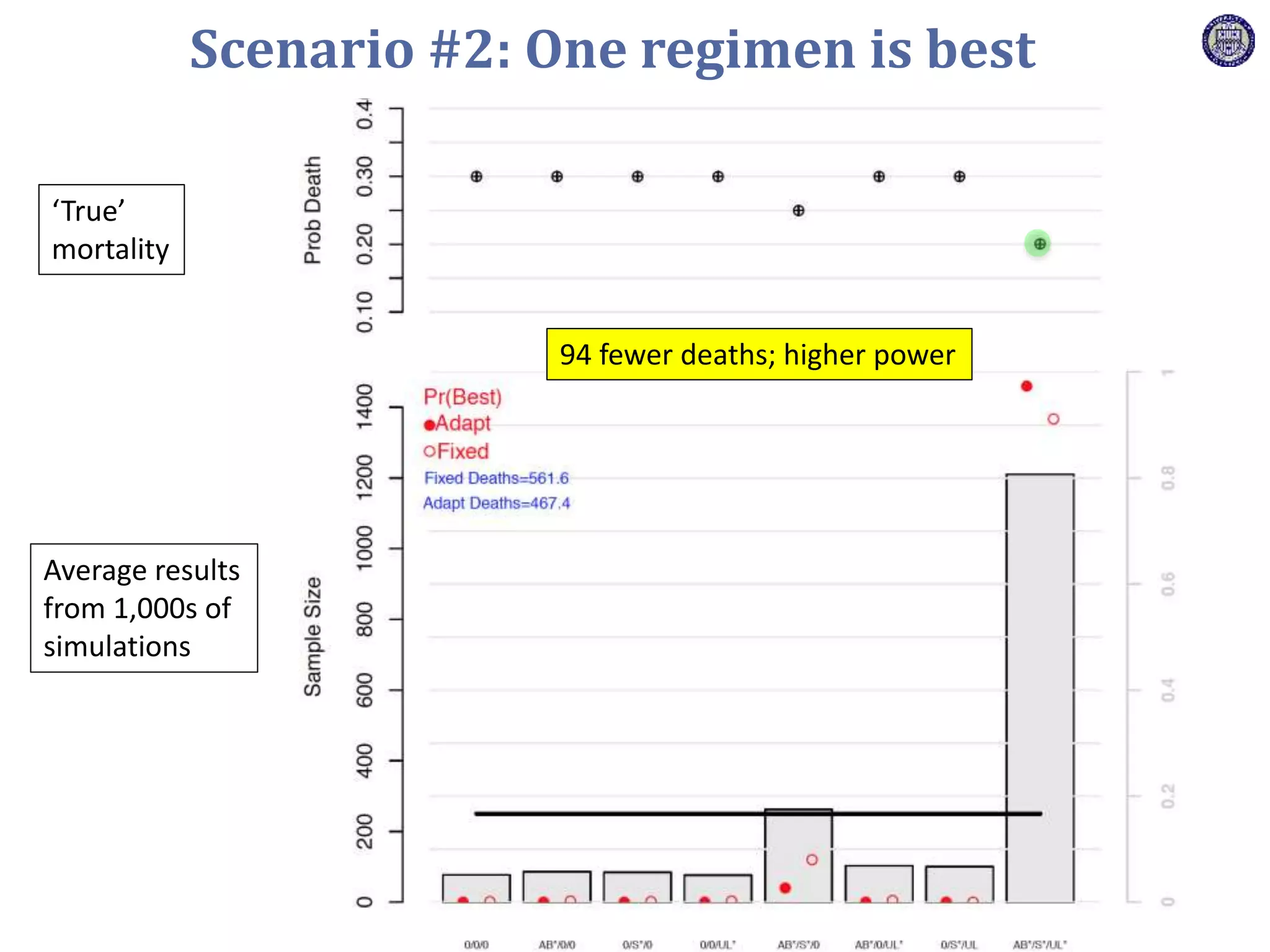
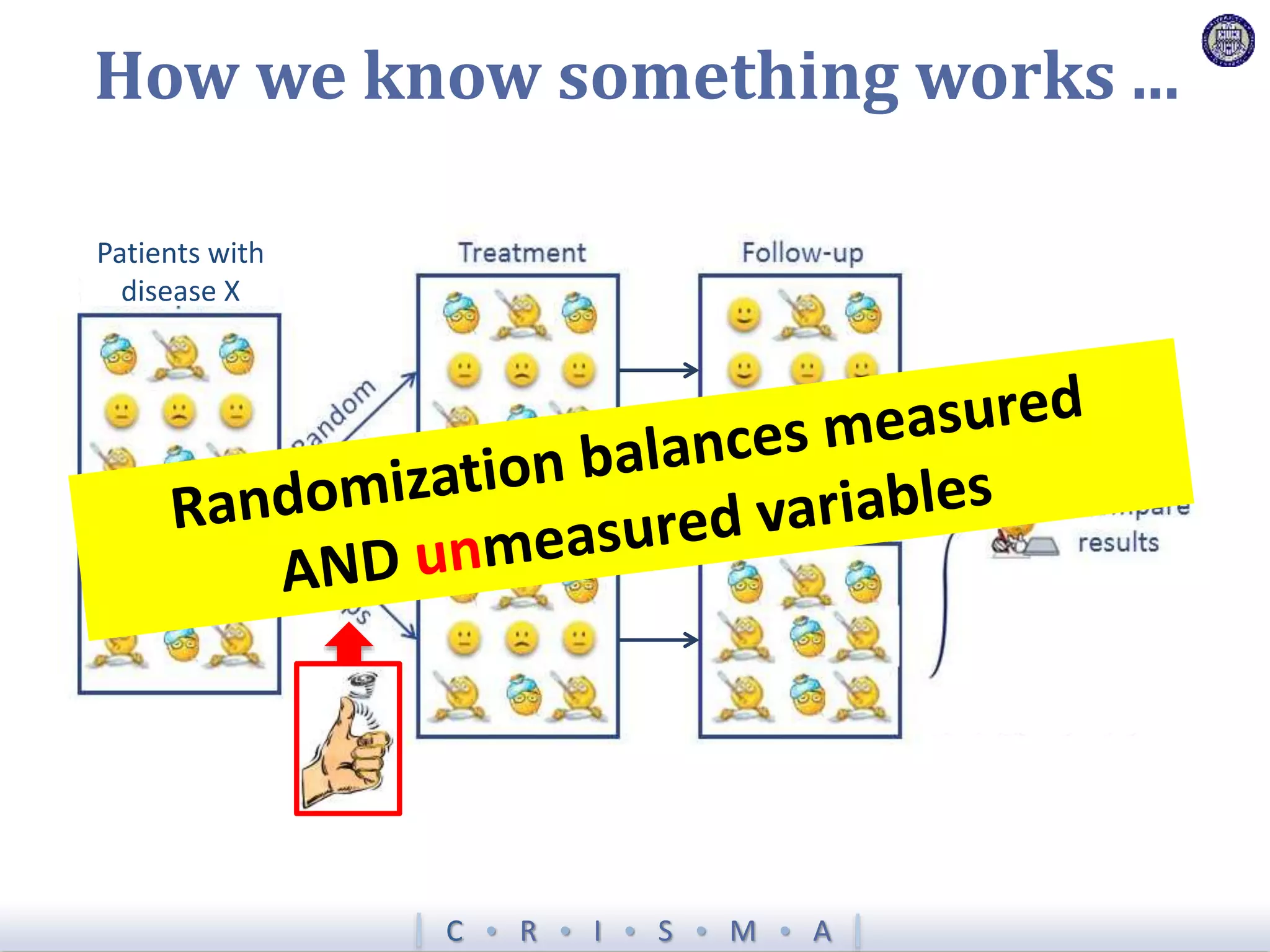
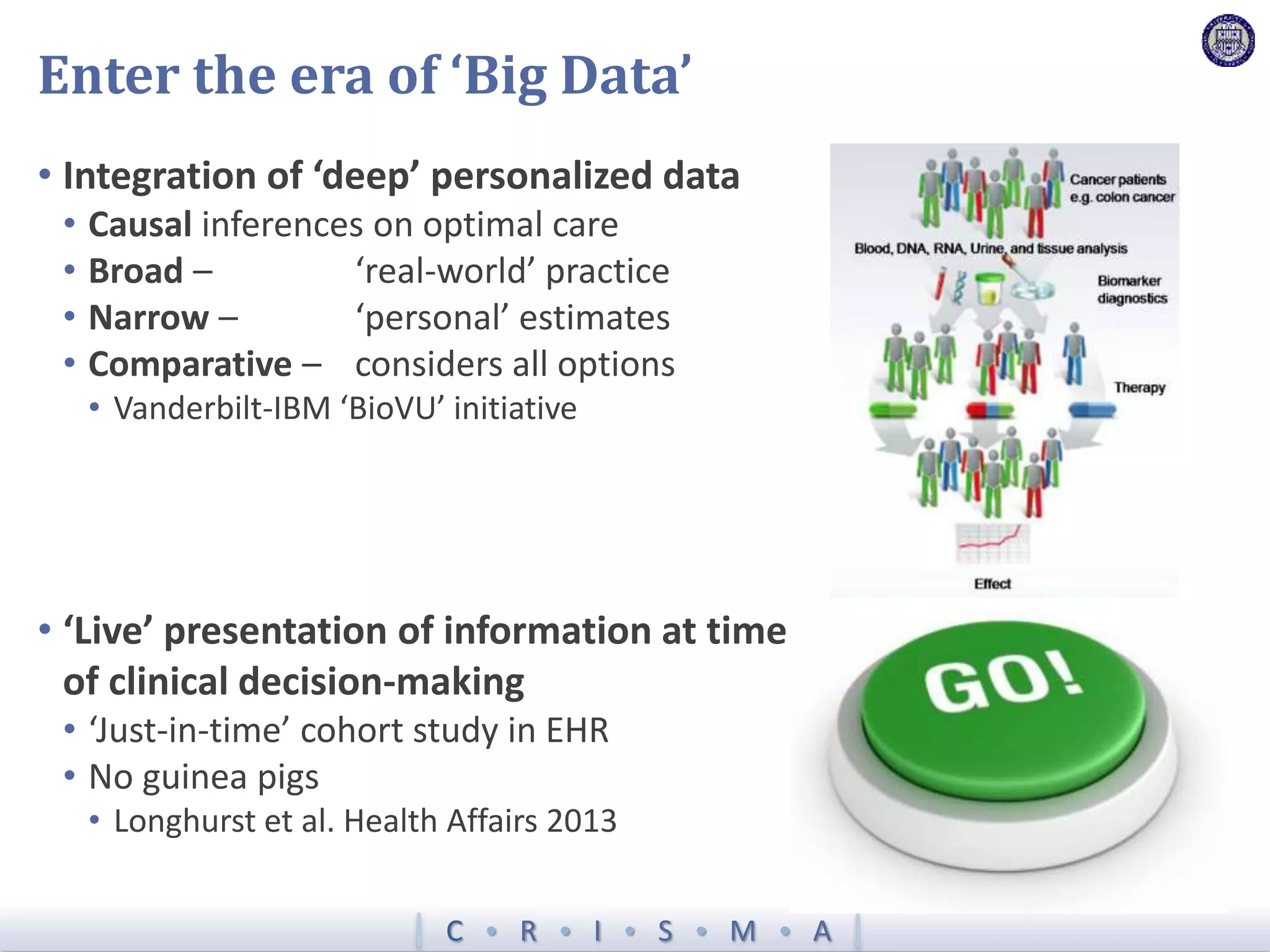
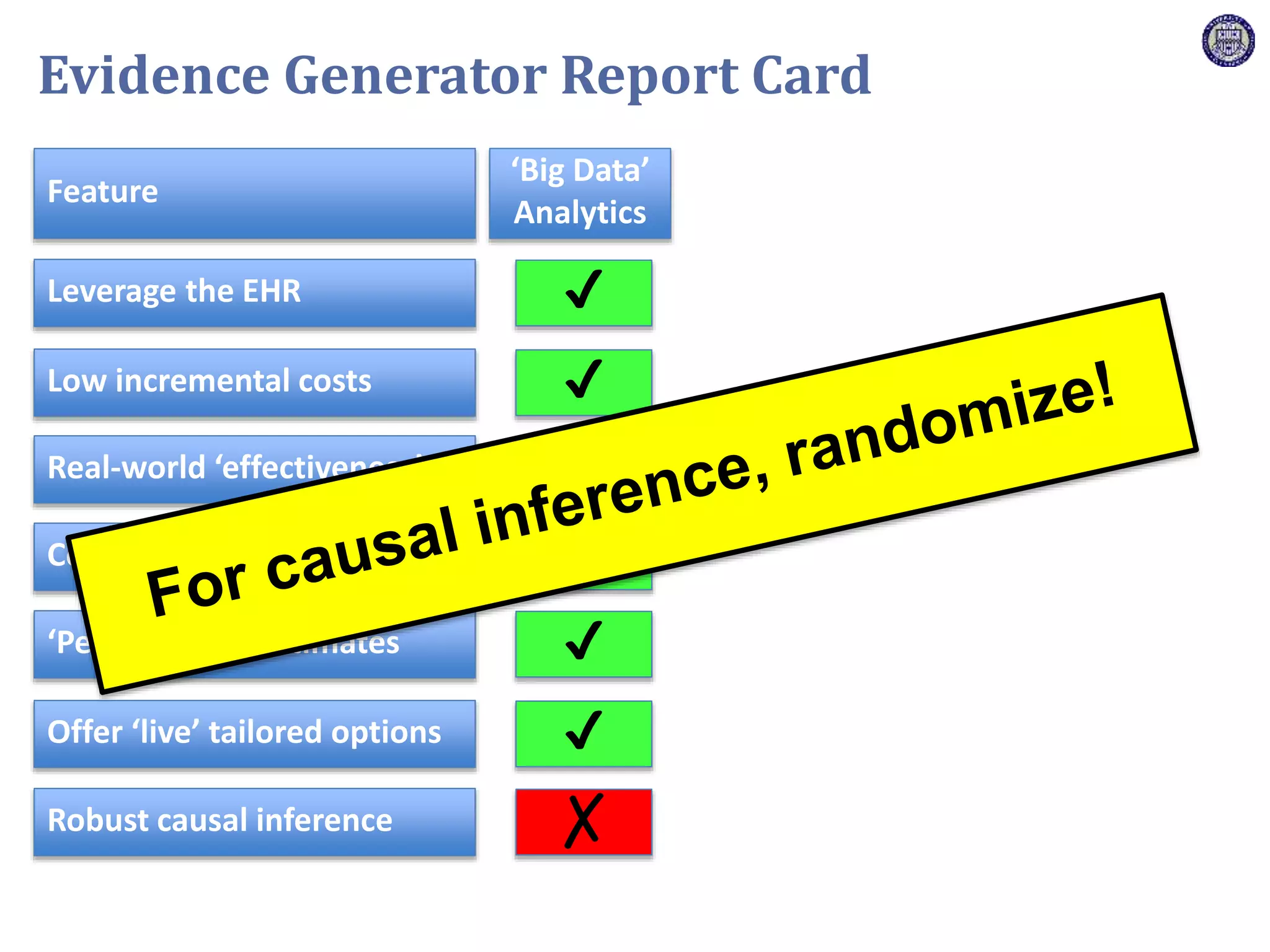
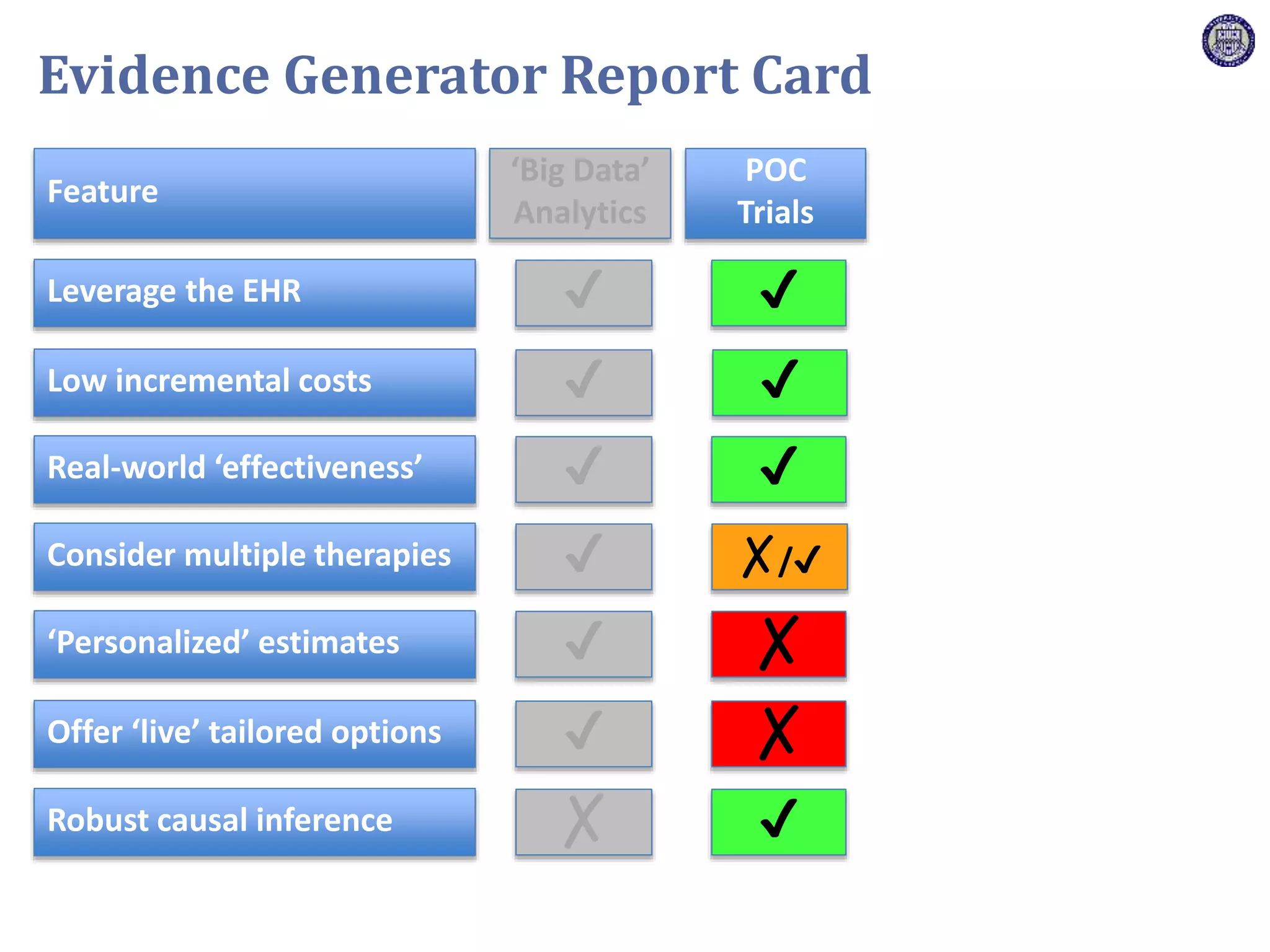
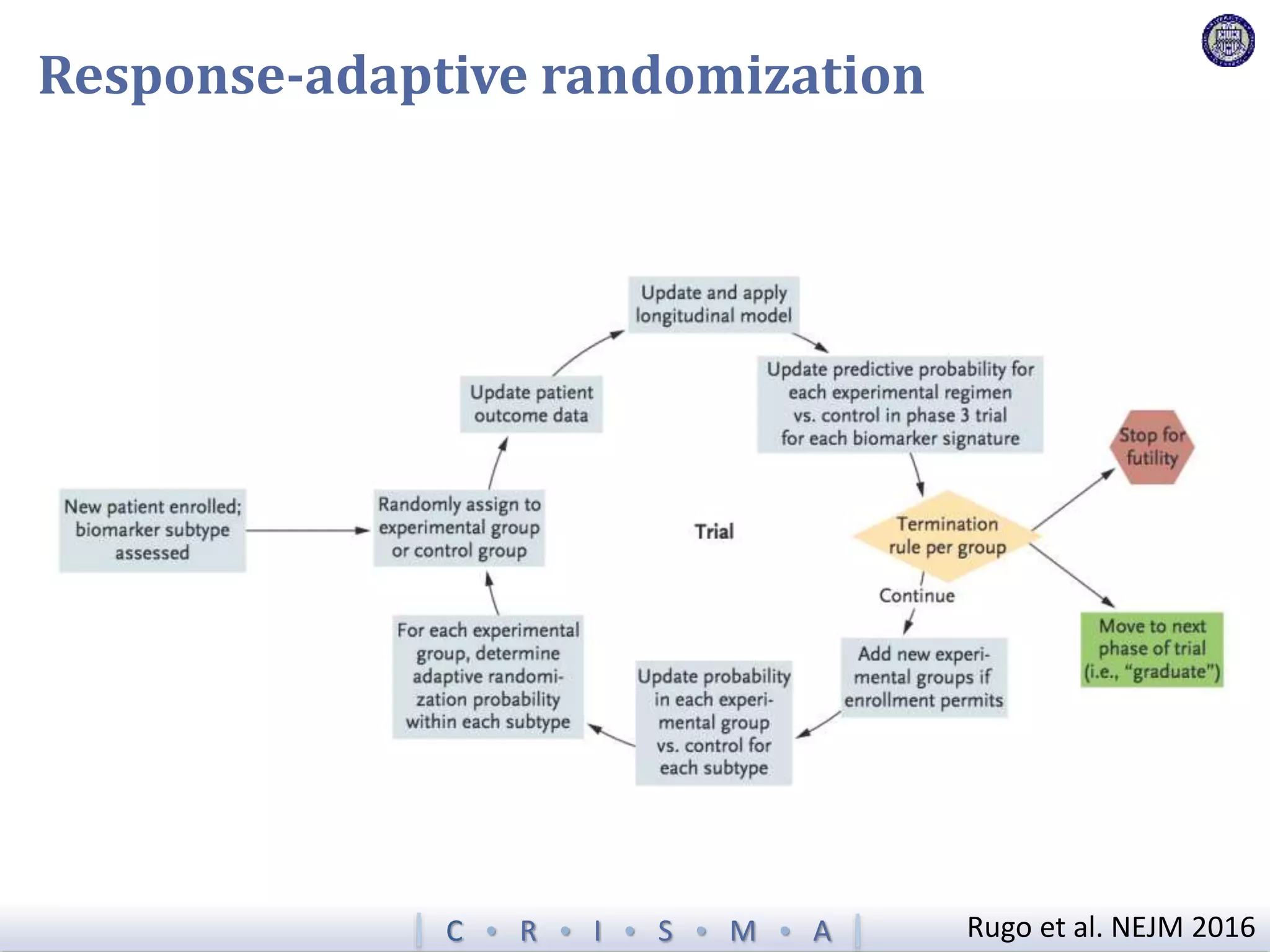
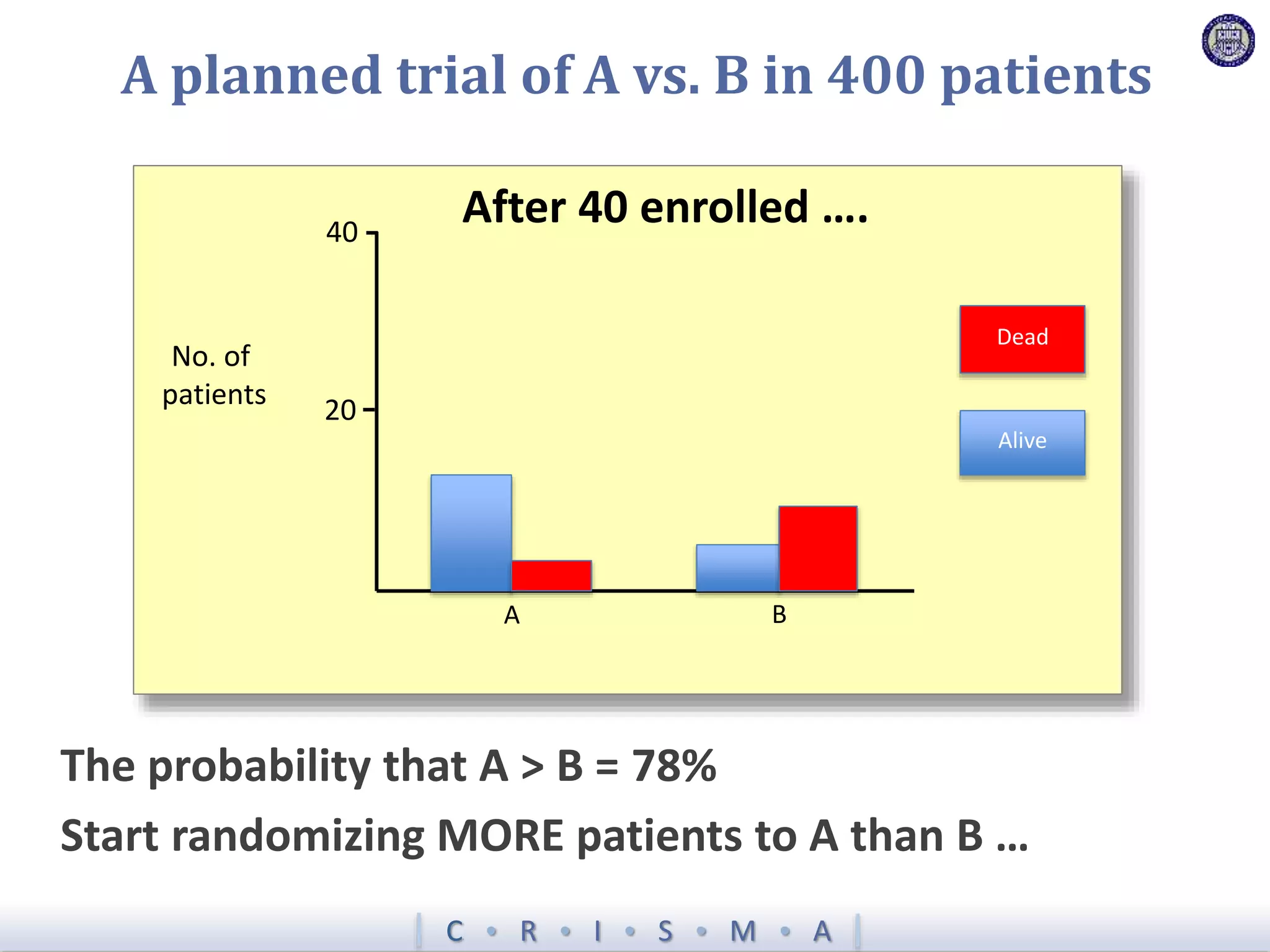
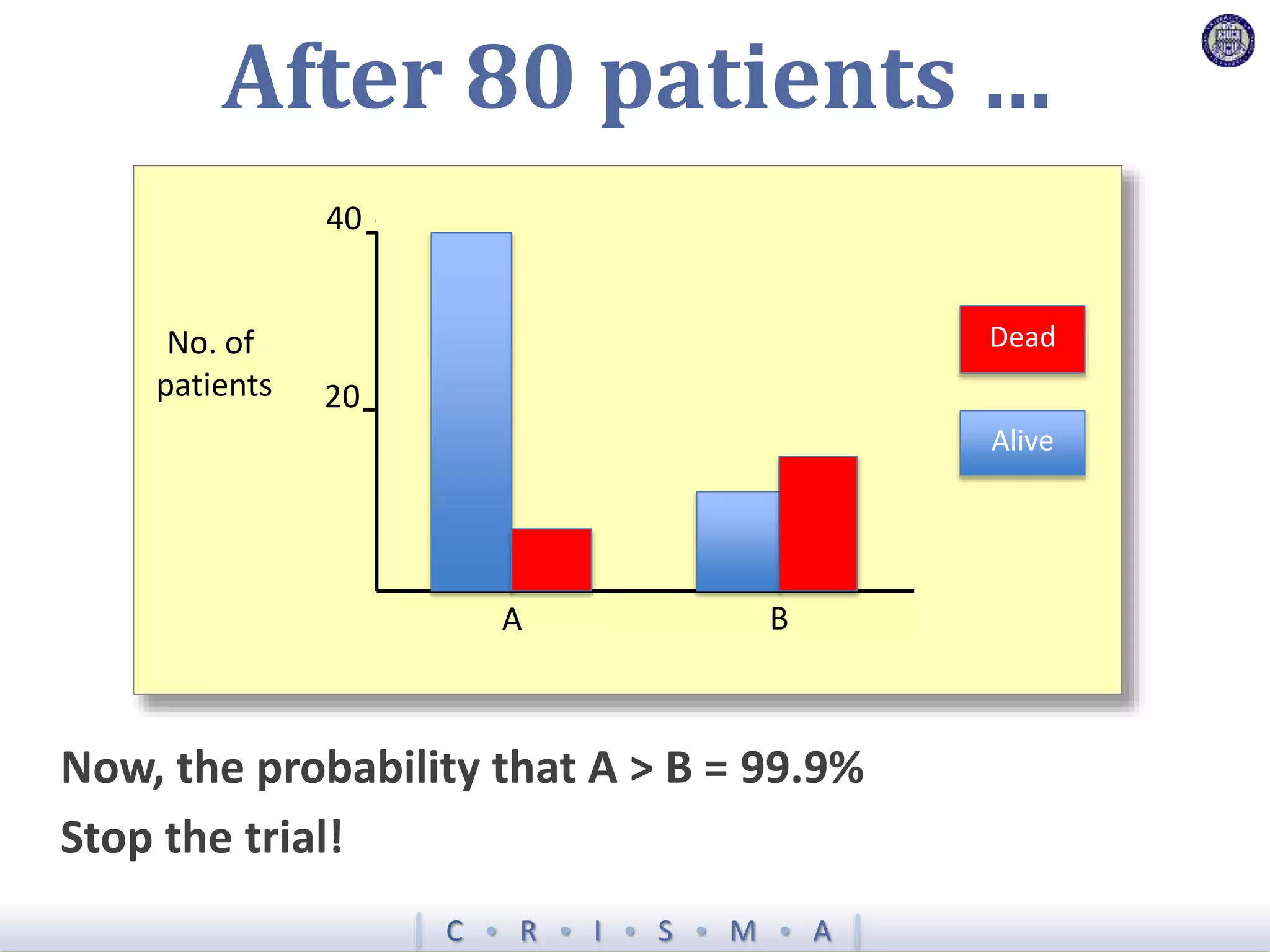
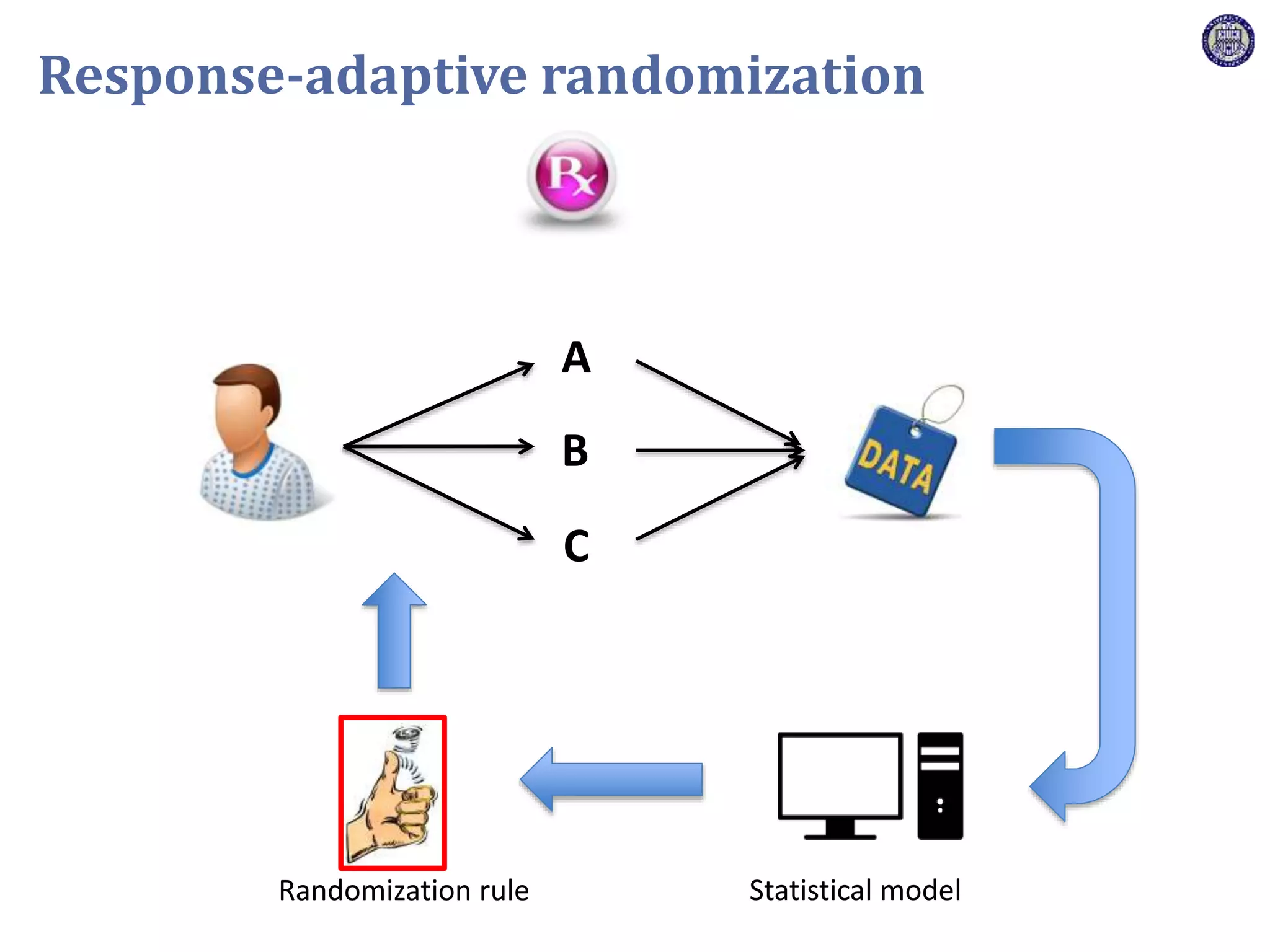
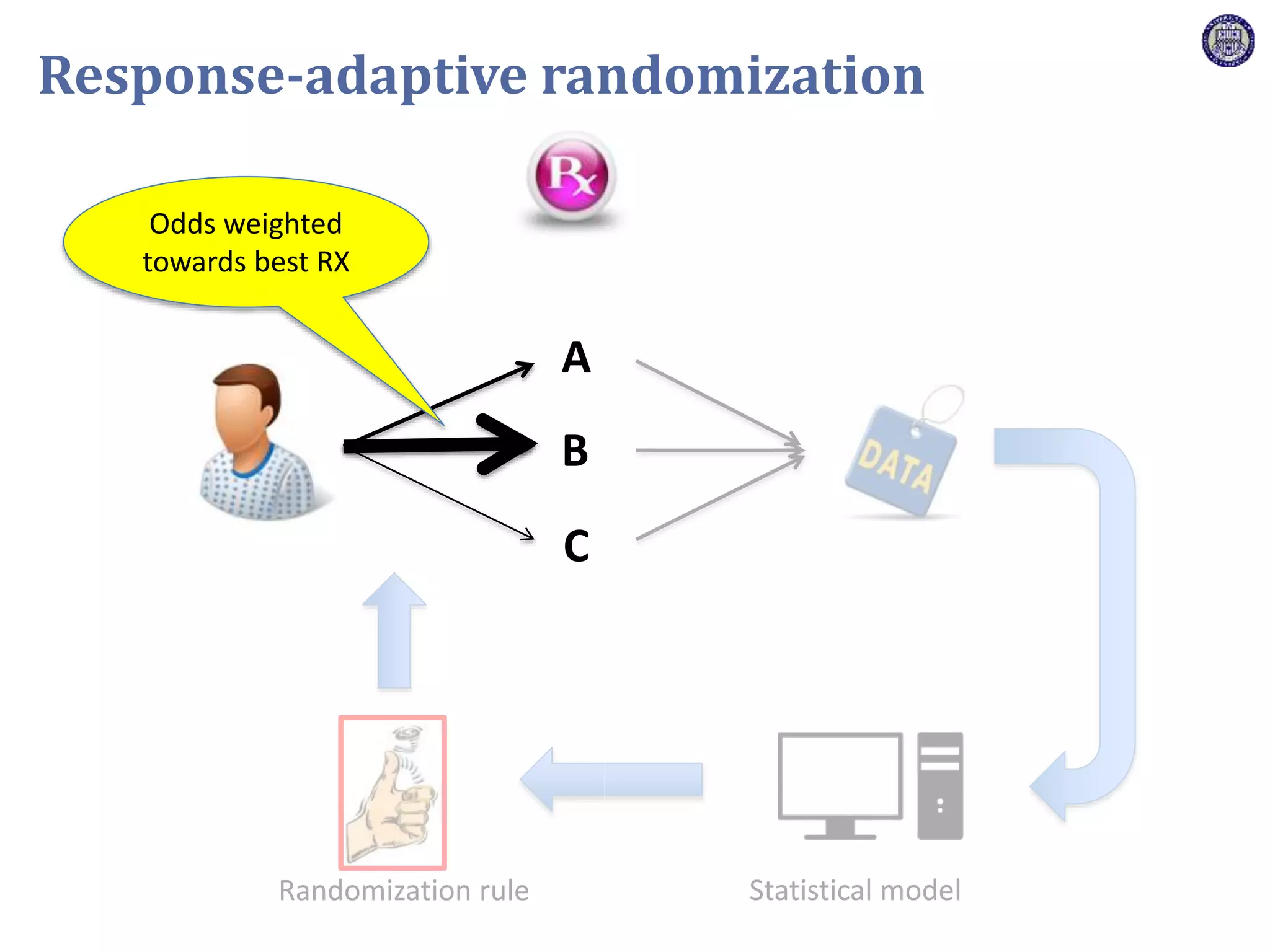
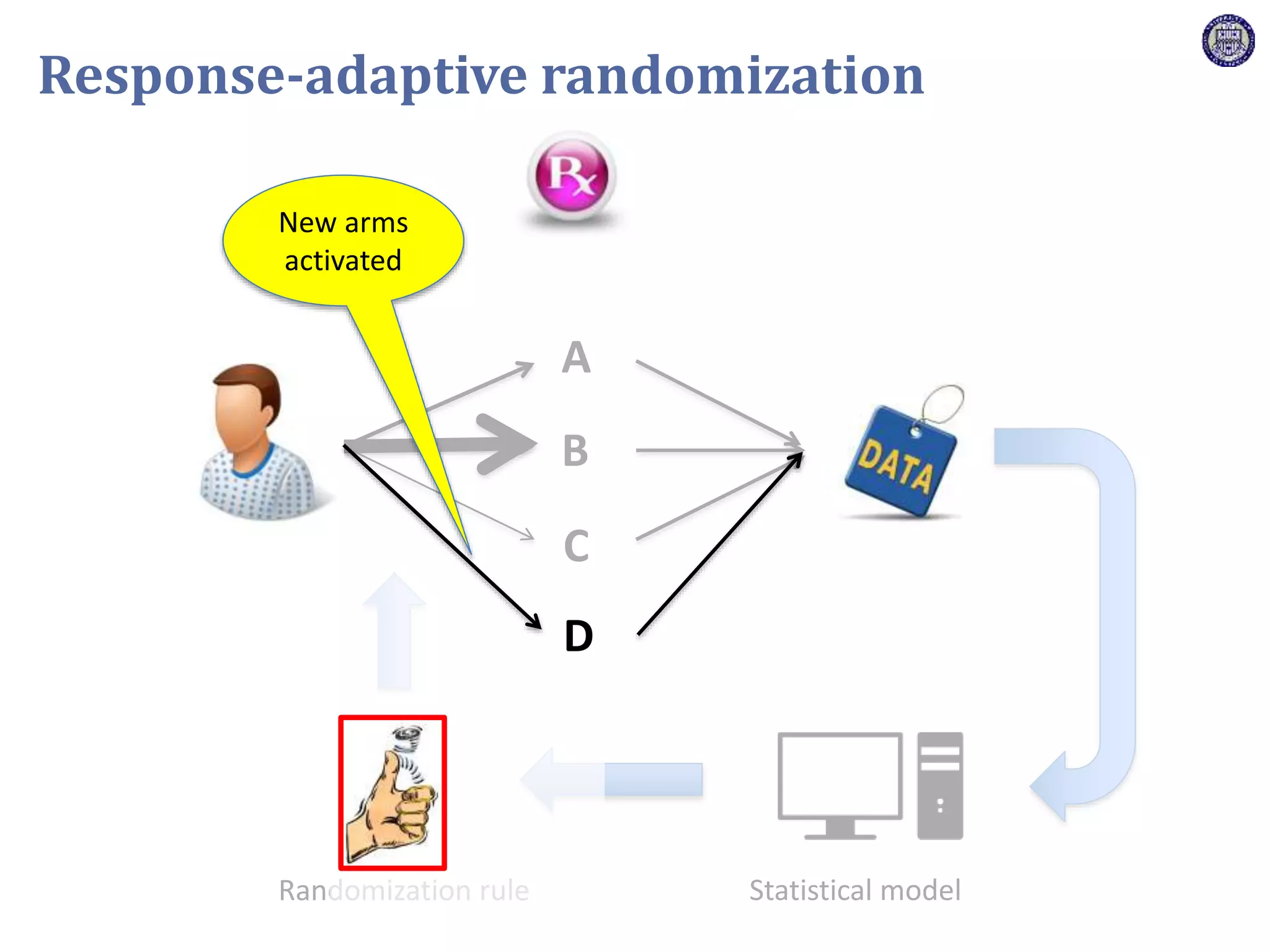
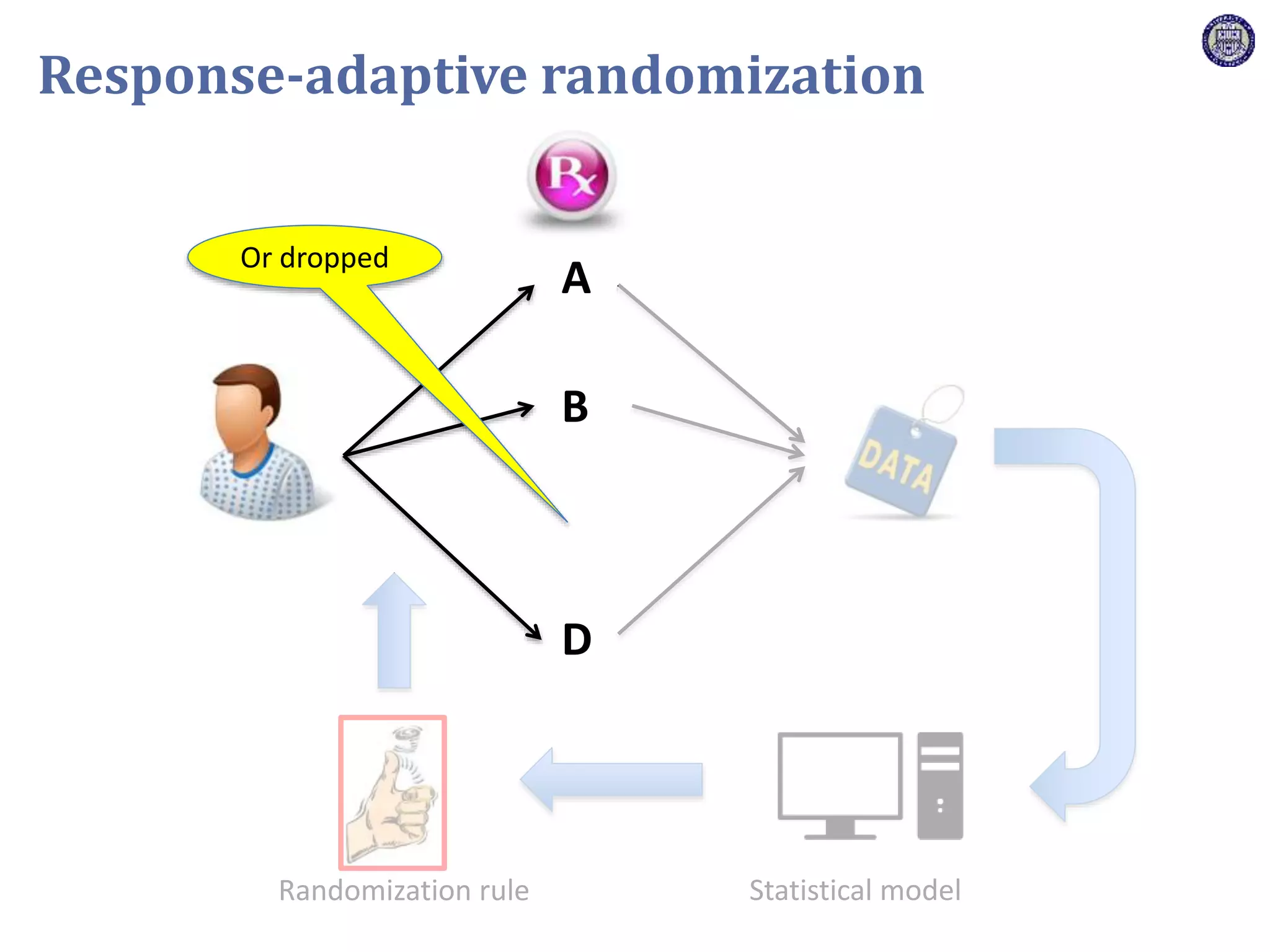
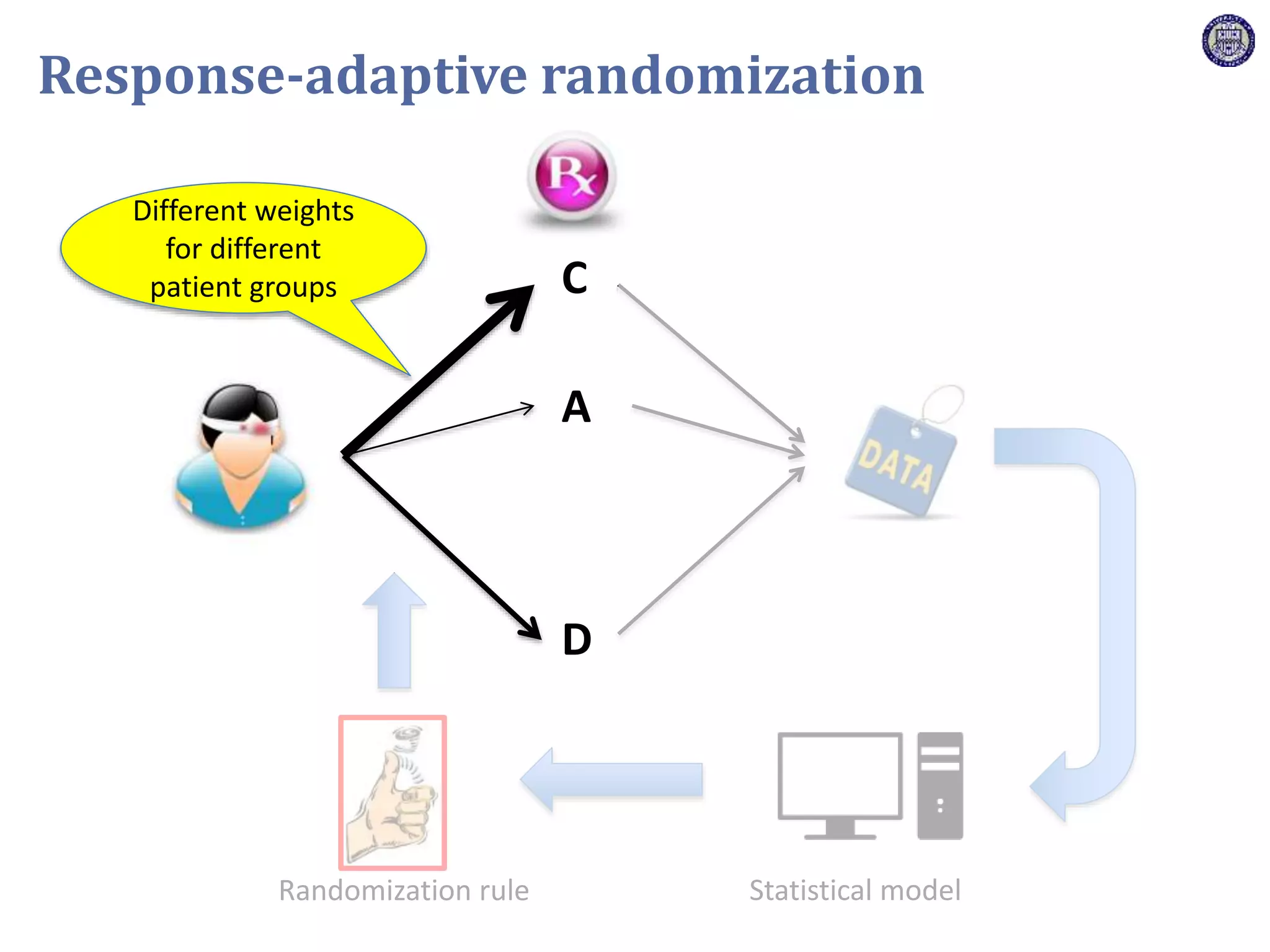
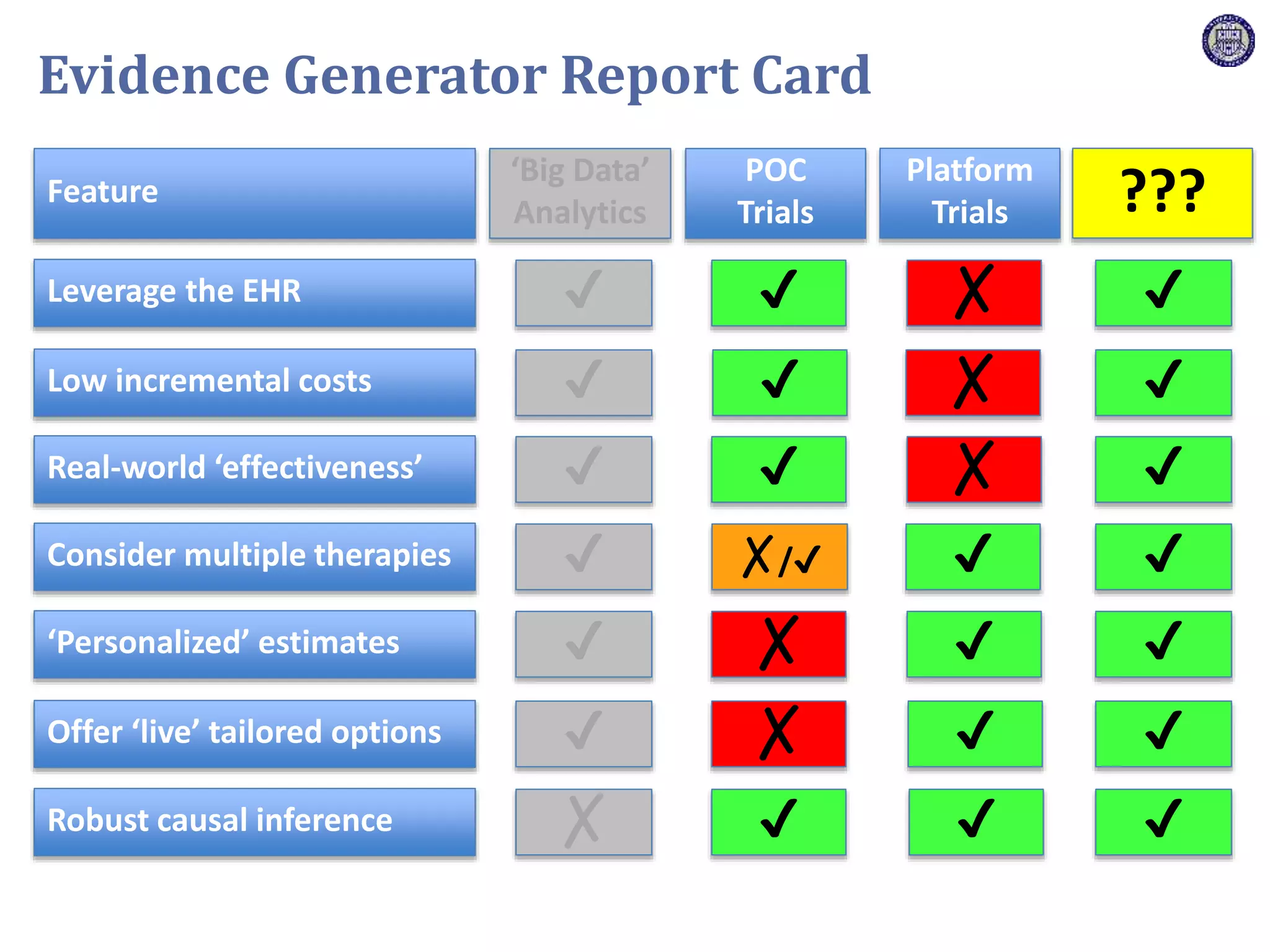
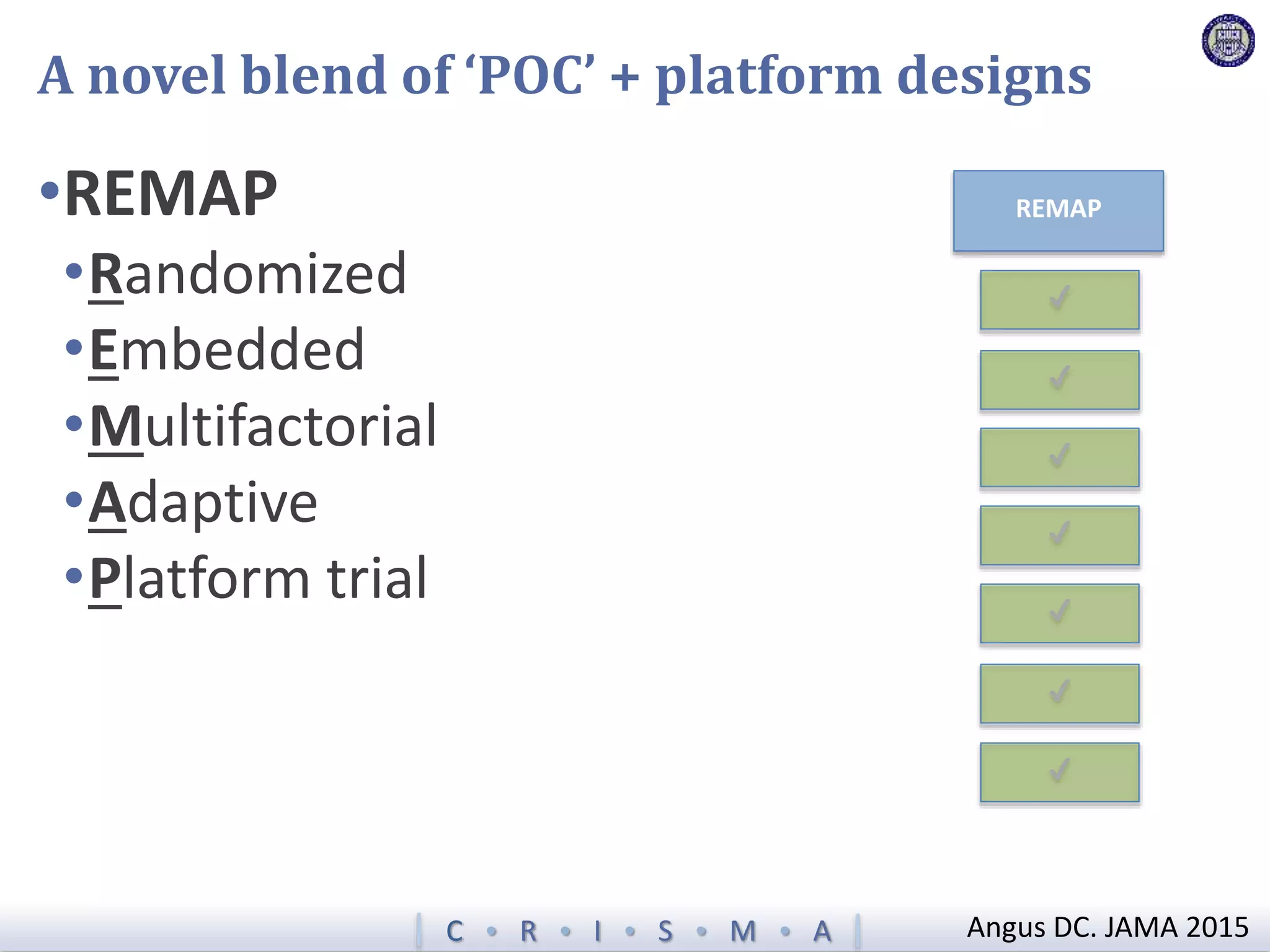
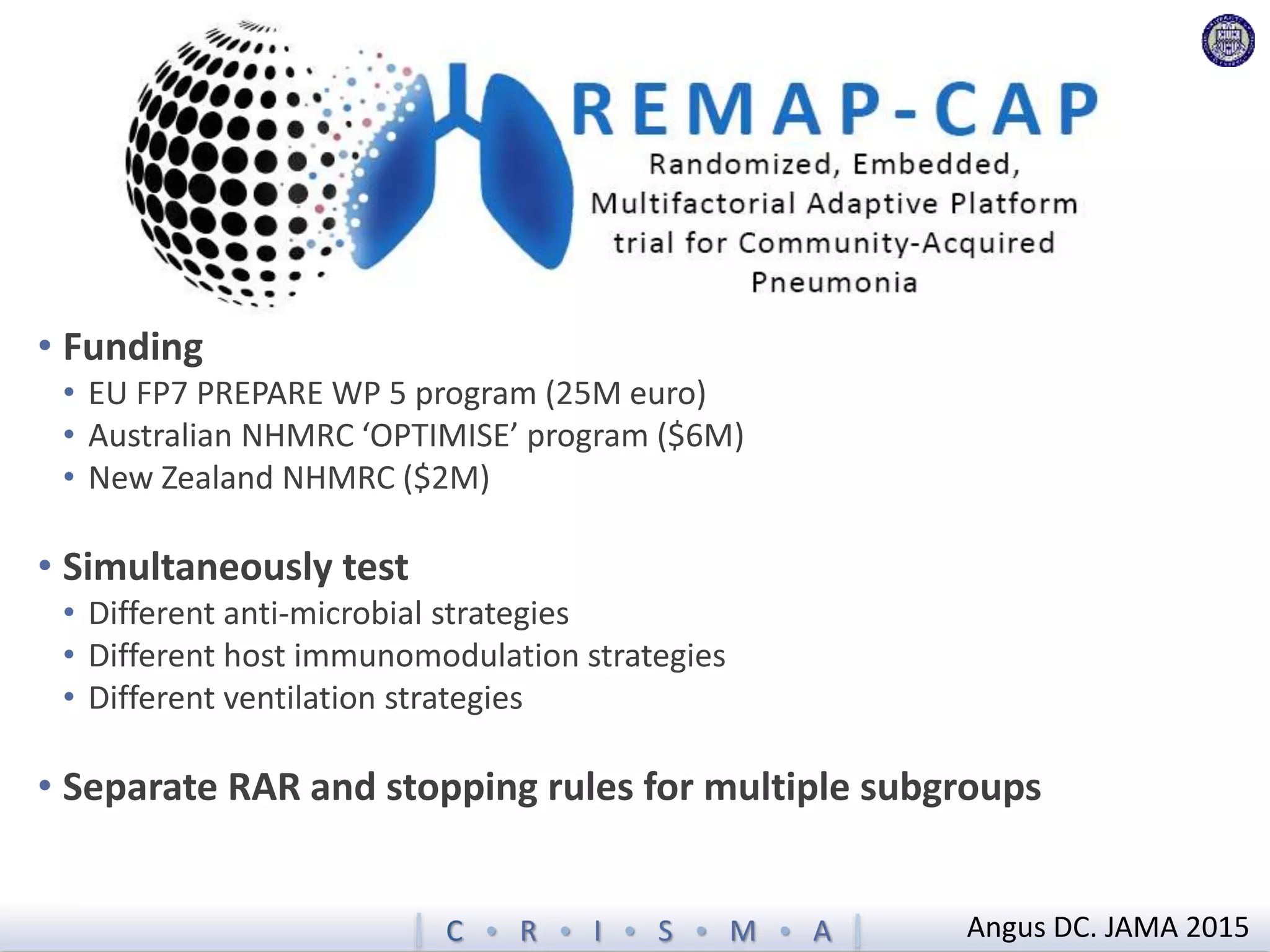
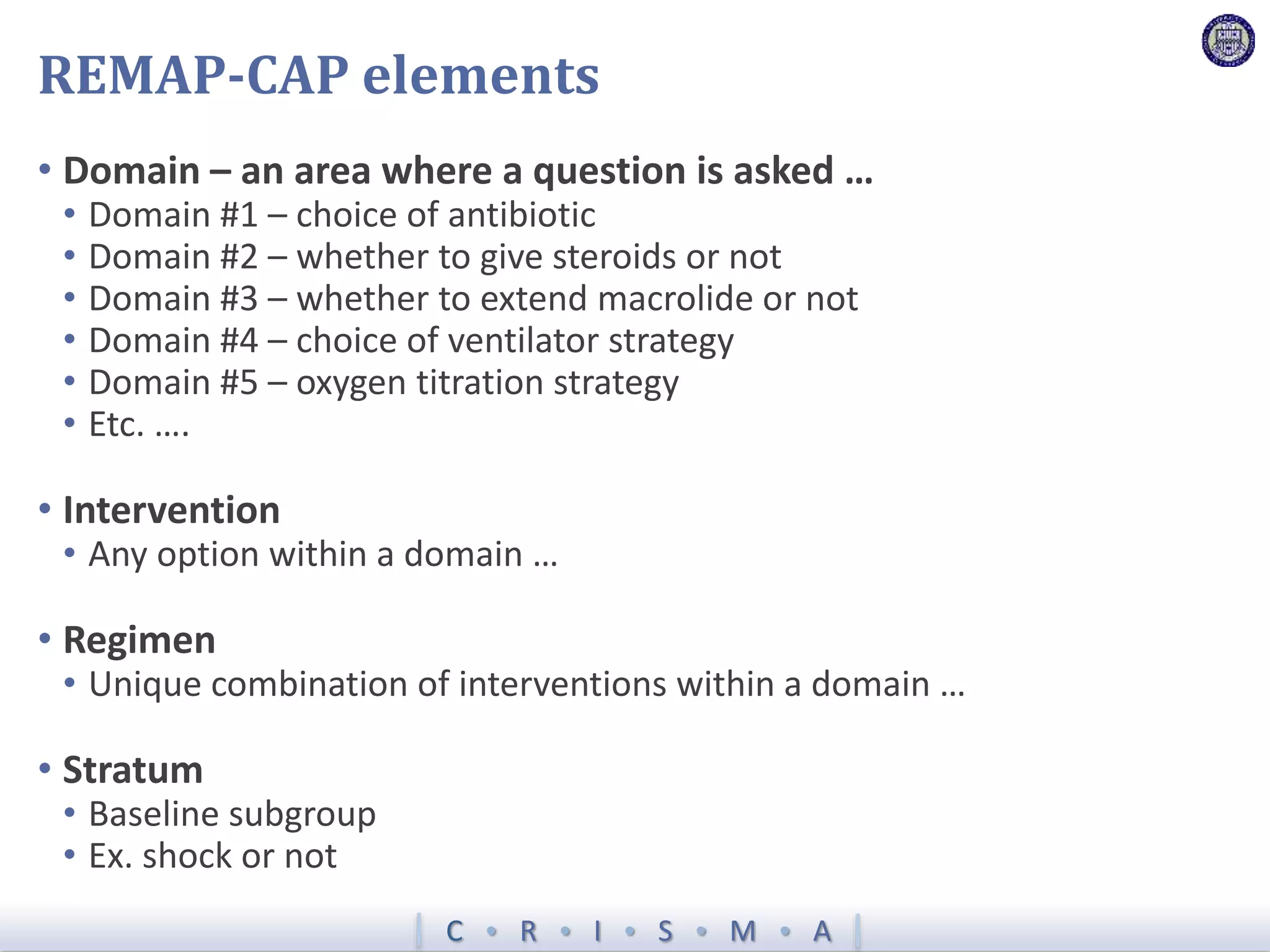
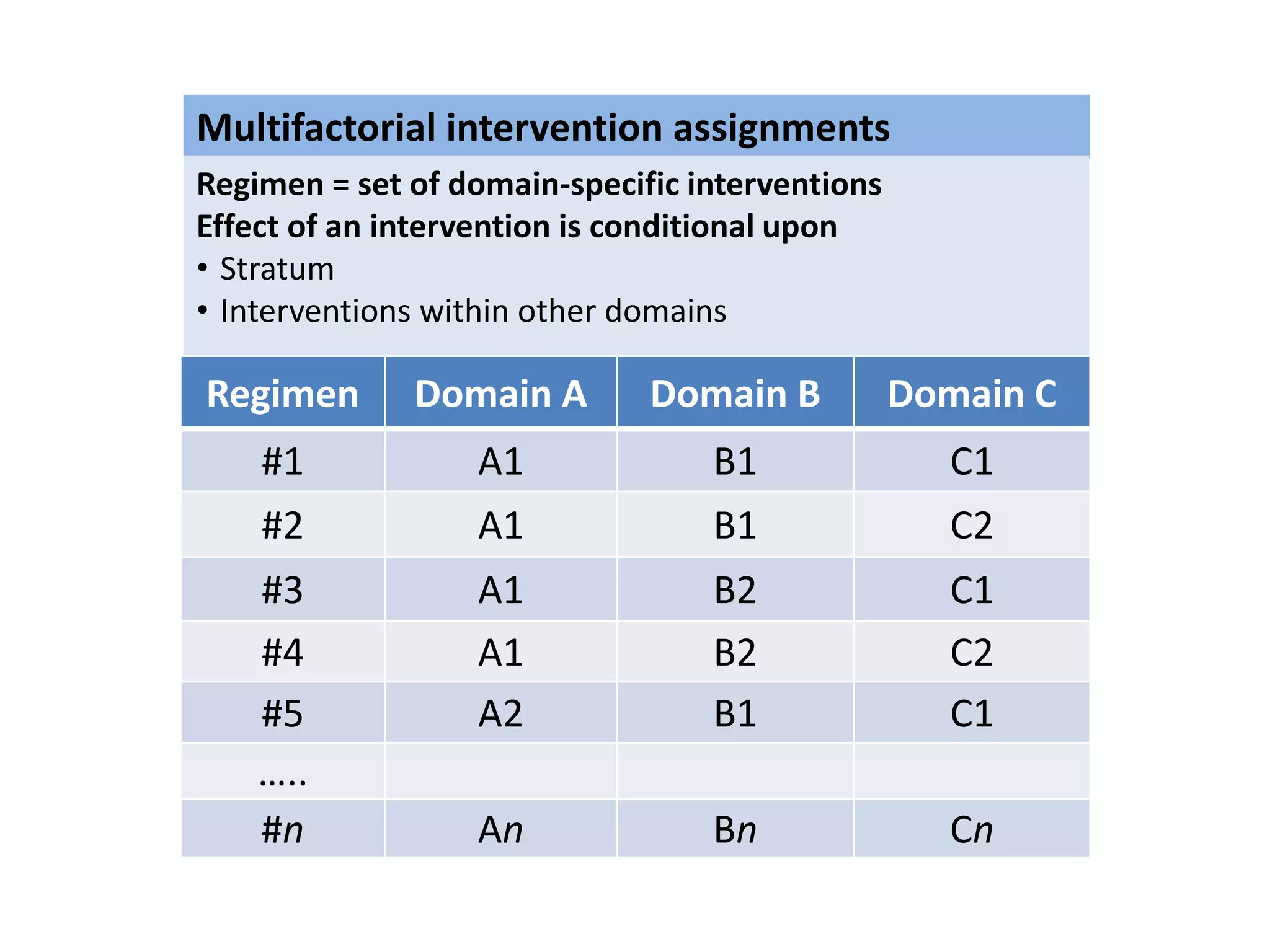
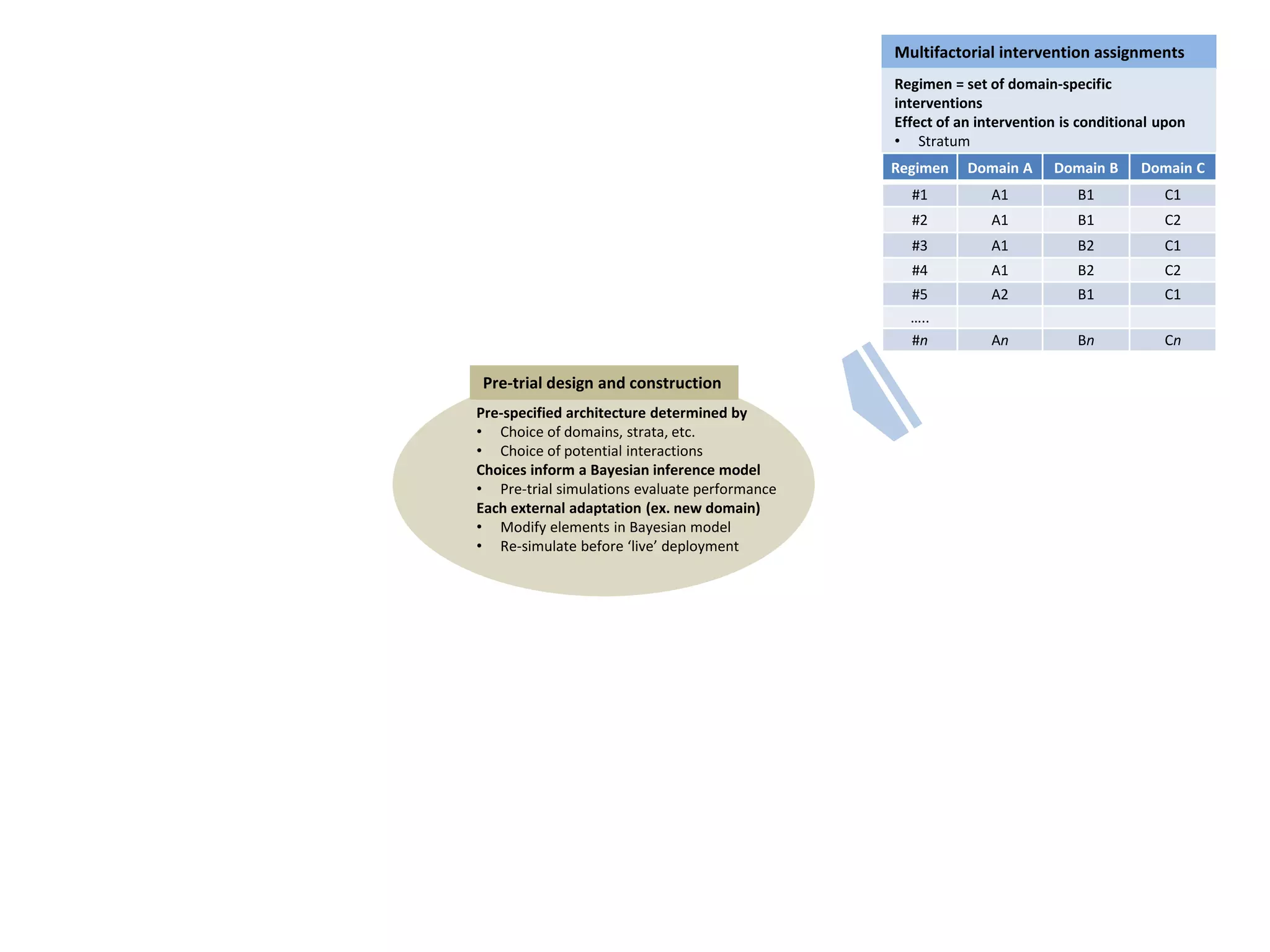
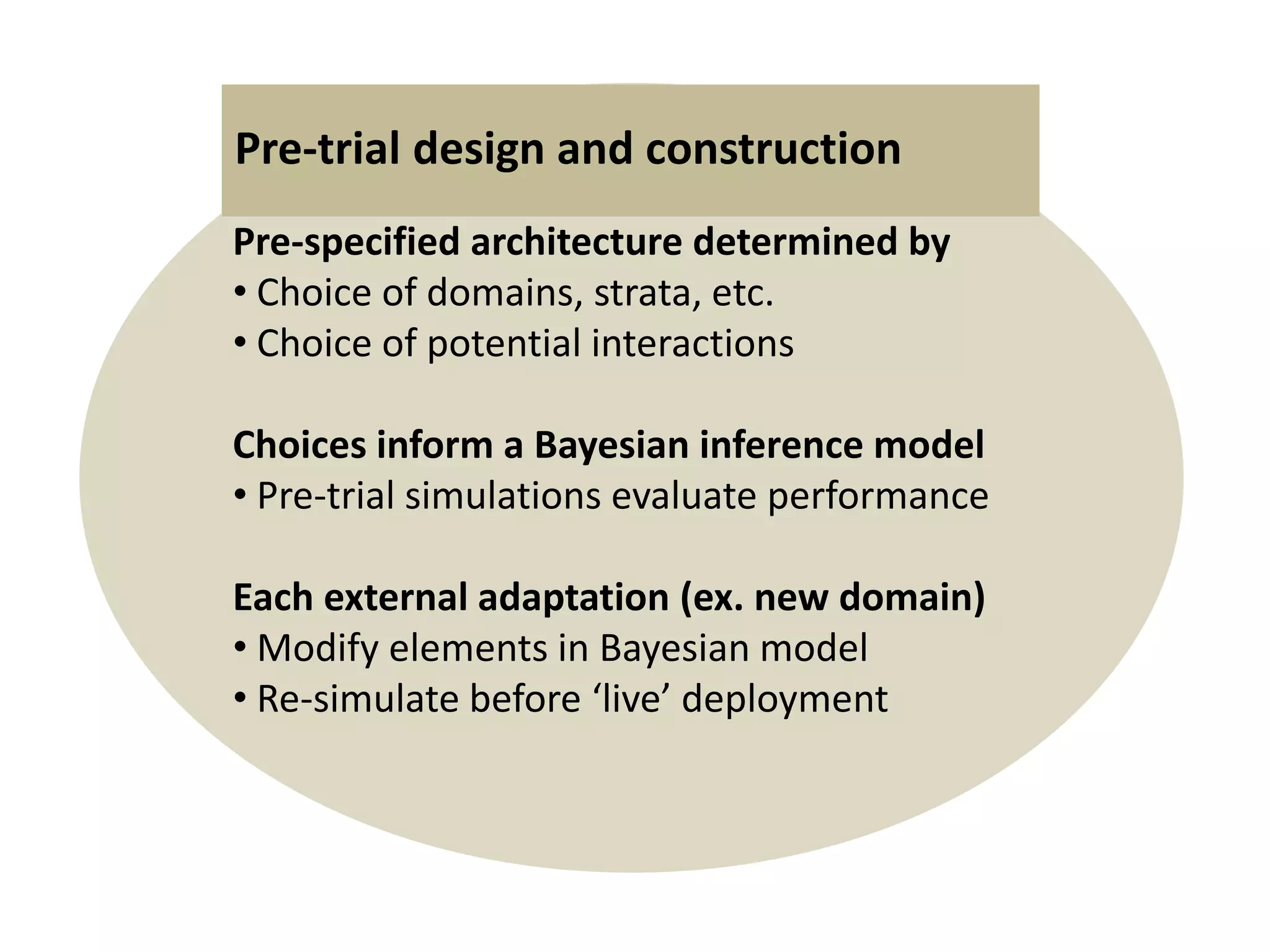
The document describes a novel platform trial design called REMAP (Randomized, Embedded, Multifactorial, Adaptive Platform) that aims to efficiently test multiple interventions for critical illness. It utilizes a point-of-care embedded design within electronic health records to rapidly enroll patients and assign multifactorial intervention regimens based on a Bayesian statistical model. The model continuously updates probabilities of intervention effectiveness based on accumulating trial data and can trigger results when an intervention is found to be superior, equivalent, or inferior for a given patient subgroup. This allows the trial to efficiently evaluate and adapt multiple treatment options in a real-world intensive care setting.












![Z = g[ ]+ R[ ]+ Time[ ]+ Interv[ ]+ Inter,Shock[ ]+ Interv,Hypox[ ]+ Interv,Interv[ ]](https://image.slidesharecdn.com/ertnvneztb61ek9fofkc-signature-8173cdb1901b6b2a92f7345b405fb7be87b1fecb83bbc56c9eadcc8941de1aa8-poli-171101062227/75/Big-data-vs-the-RCT-Derek-Angus-SSAI2017-40-2048.jpg)