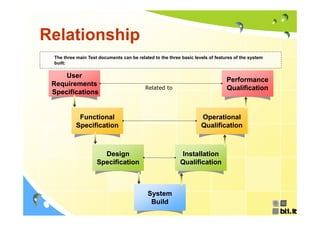
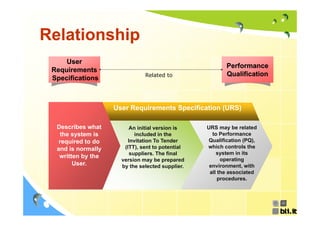
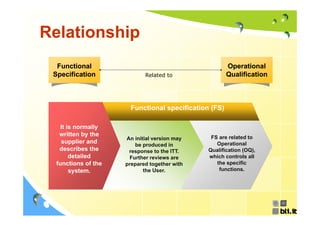
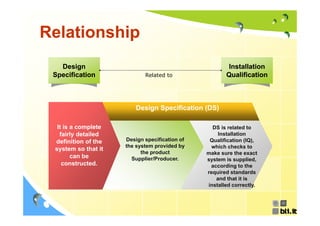
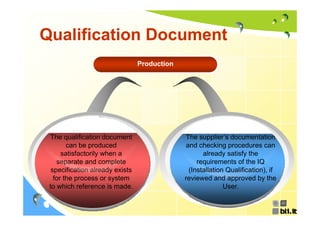
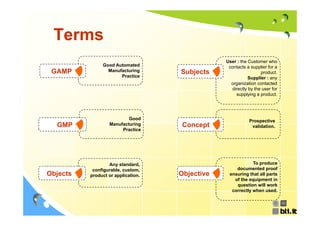
This document discusses concepts regarding the GAMP Guide. It provides an overview of key terms like GAMP and GMP. It outlines the origin of the GAMP Guide in the early 1990s to improve understanding of pharmaceutical regulation and validation. It describes the general validation process activities of planning, specifications, test planning, testing, and review. It explains the relationship between user requirements, functional specifications, design specifications, and the different qualification documents. Finally, it acknowledges the reader.



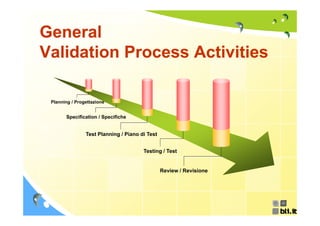
![General
Validation Process Activities
Planning
[progettazione]
Written Validation Plan
(Piano di Validazione).](https://image.slidesharecdn.com/bli-it-concepts-regarding-gamp-guide-en-120405050728-phpapp02/85/Bli-it-concepts-regarding-gamp-guide-en-7-320.jpg)
![General
Validation Process Activities
Specifications
[specifiche]
Specify and agree what is required.
required](https://image.slidesharecdn.com/bli-it-concepts-regarding-gamp-guide-en-120405050728-phpapp02/85/Bli-it-concepts-regarding-gamp-guide-en-8-320.jpg)
![General
Validation Process Activities
Test Planning IQ [installation qualification]
OQ [operational qualification]
[piano di test] PQ [performance qualification]
Prepare document to describe how the system is to be
tested.](https://image.slidesharecdn.com/bli-it-concepts-regarding-gamp-guide-en-120405050728-phpapp02/85/Bli-it-concepts-regarding-gamp-guide-en-9-320.jpg)
![General Validation Process
Activities
Testing IQ [installation qualification]
OQ [operational qualification]
[test] PQ [performance qualification]
Perform tests and collect results
results.](https://image.slidesharecdn.com/bli-it-concepts-regarding-gamp-guide-en-120405050728-phpapp02/85/Bli-it-concepts-regarding-gamp-guide-en-10-320.jpg)
![General Validation
G l V lid ti
Process Activities
Review
[revisione]
Review results to show that the system performs as
specified plus any reservations.](https://image.slidesharecdn.com/bli-it-concepts-regarding-gamp-guide-en-120405050728-phpapp02/85/Bli-it-concepts-regarding-gamp-guide-en-11-320.jpg)