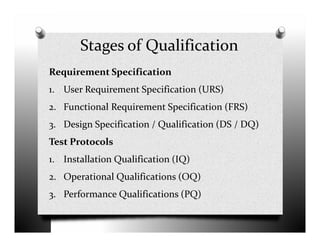
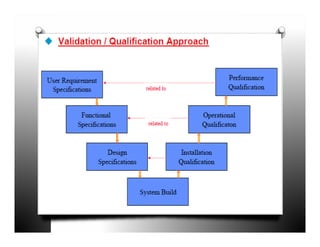
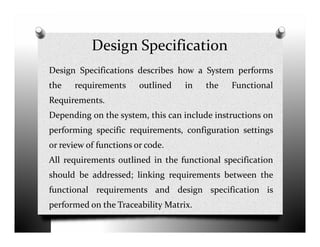
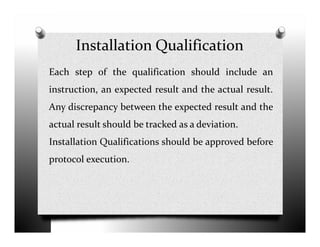
The document discusses validation and project management. It defines validation as proving that a process will consistently produce expected results. Validation involves qualification and testing stages like installation qualification and operational qualification. A requirement traceability matrix links requirements throughout the validation process to ensure all are tested. Deviations from expected test results are tracked, and a validation summary report provides an overview of the completed validation project. Change control manages how changes are introduced to validated systems.