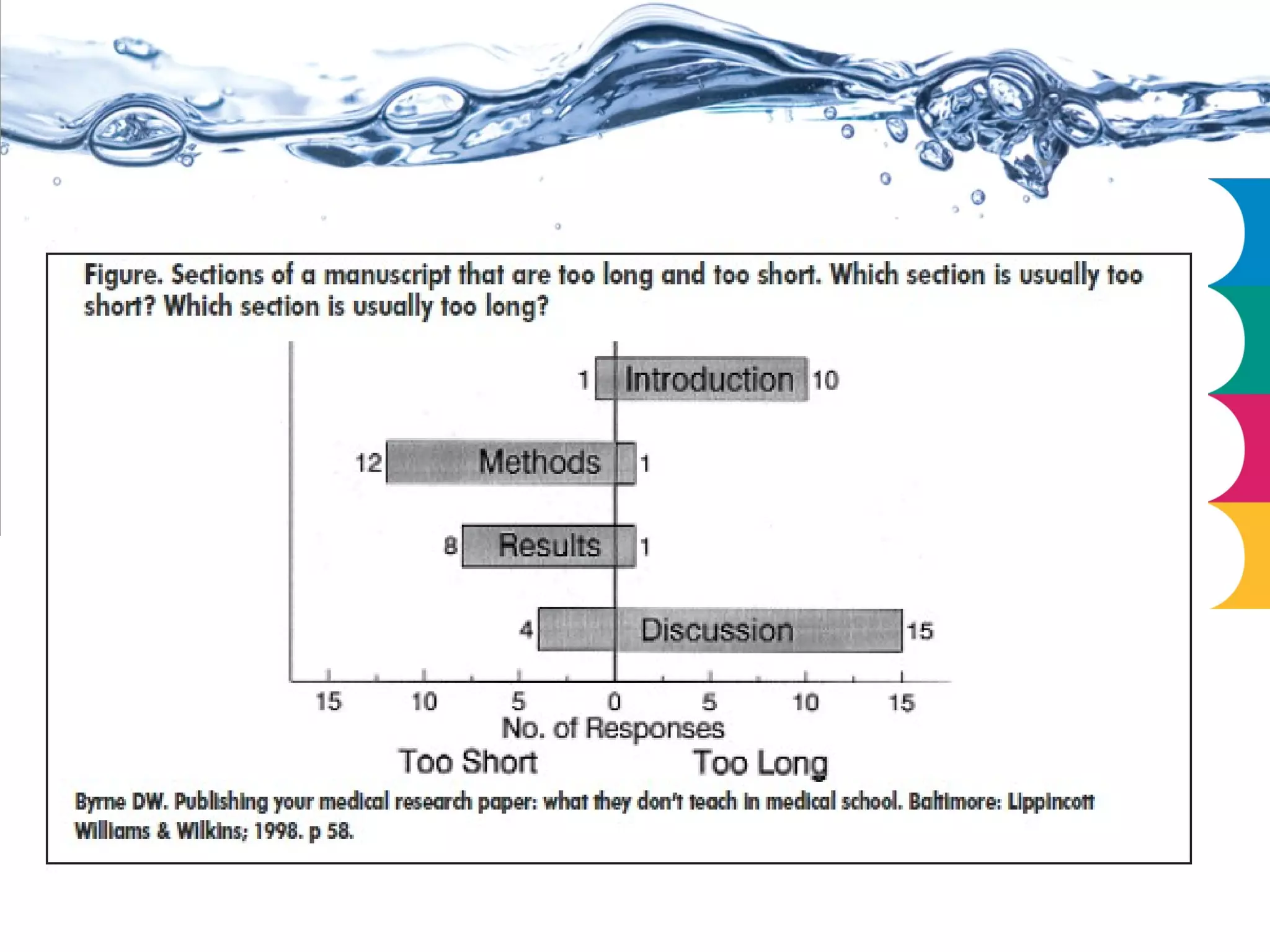
This document outlines the essential requirements and ethical considerations for preparing a manuscript for submission to biomedical journals, emphasizing clarity, conciseness, and adherence to specific guidelines. It details the 'IMRAD' structure for organization, the importance of thorough referencing, and the handling of conflicts of interest. Additionally, it addresses common questions and provides resources for authors on manuscript preparation and submission processes.