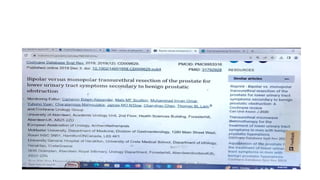
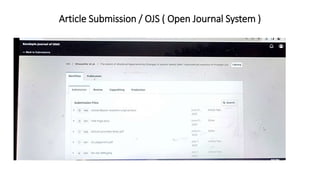
The document provides a comprehensive guide on writing a research paper, including objectives, structure, terminology, citation methods, and the role of technology in research. It outlines the IMRaD format for structuring articles, discusses various types of journals, and highlights the importance of ethical considerations like consent and conflict of interest. Additionally, it offers tips for effective literature review, data collection, and ensures proper referencing according to required styles.





















![References
• Can be written as Superscript BPH 2 , Superscript with Brackets (9) , Plain Brackets
[ 9 ]
• MLA ( Modern Language Association ) system Humanities and Arts
• APA (American Psychological Association ) Education and Psychology
• Harvard system
• Vancouver Style ( Medicine and Health Sciences ) / NLM ( National Library of
Medicine ) / Author-Number System / AMA ( American Medical Association )
• MHRA ( Modern Humanities Research Association ) System](https://image.slidesharecdn.com/writingaresearchpaper-230901190413-bc943fc7/85/Writing-a-Research-Paper-pptx-27-320.jpg)